Specificity requirements for human telomere protein interaction with telomerase holoenzyme
- PMID: 22893708
- PMCID: PMC3464550
- DOI: 10.1074/jbc.M112.394767
Specificity requirements for human telomere protein interaction with telomerase holoenzyme
Abstract
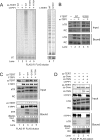
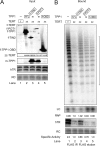
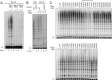
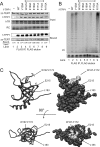
Human telomeres are maintained by the enzyme telomerase, which uses a template within its integral RNA subunit (hTR) and telomerase reverse transcriptase protein (TERT) to accomplish the synthesis of single-stranded DNA repeats. Many questions remain unresolved about the cellular regulation of telomerase subunits and the fully assembled telomerase holoenzyme, including the basis for the specificity of binding and acting on telomeres. Previous studies have revealed that the telomere protein TPP1 is necessary for stable TERT and hTR association with telomeres in vivo. Here, we expand the biochemical characterization and understanding of TPP1 interaction with TERT and the catalytically active telomerase holoenzyme. Using extracts from human cells, we show that TPP1 interacts sequence-specifically with TERT when TERT is assembled into holoenzyme context. In holoenzyme context, the TERT N-terminal domain mediates a TPP1 interaction. Assays of stable subunit complexes purified after their cellular assembly suggest that other telomere proteins do not necessarily influence TPP1 association with telomerase holoenzyme or alter its impact on elongation processivity. We show that a domain of recombinant TPP1 comprised of an oligonucleotide/oligosaccharide binding fold recapitulates the full-length protein interaction specificity for the TERT N-terminal domain assembled into telomerase holoenzyme. By global analysis of TPP1 side chain requirements for holoenzyme association, we demonstrate a selective requirement for the amino acids in one surface-exposed protein loop. Our results reveal the biochemical determinants of a sequence-specific TPP1-TERT interaction in human cells, with implications for the mechanisms of TPP1 function in recruiting telomerase subunits to telomeres and in promoting telomere elongation.
Figures


Similar articles
-
TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends.Cell. 2012 Aug 3;150(3):481-94. doi: 10.1016/j.cell.2012.07.012. Cell. 2012. PMID: 22863003 Free PMC article.
-
Shared Subunits of Tetrahymena Telomerase Holoenzyme and Replication Protein A Have Different Functions in Different Cellular Complexes.J Biol Chem. 2017 Jan 6;292(1):217-228. doi: 10.1074/jbc.M116.763664. Epub 2016 Nov 28. J Biol Chem. 2017. PMID: 27895115 Free PMC article.
-
The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity.Nature. 2012 Dec 13;492(7428):285-9. doi: 10.1038/nature11648. Epub 2012 Oct 24. Nature. 2012. PMID: 23103865 Free PMC article.
-
Multiple facets of TPP1 in telomere maintenance.Biochim Biophys Acta. 2014 Sep;1844(9):1550-9. doi: 10.1016/j.bbapap.2014.04.014. Epub 2014 Apr 26. Biochim Biophys Acta. 2014. PMID: 24780581 Free PMC article. Review.
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
Cited by
-
Contributions of the TEL-patch amino acid cluster on TPP1 to telomeric DNA synthesis by human telomerase.J Mol Biol. 2015 Mar 27;427(6 Pt B):1291-1303. doi: 10.1016/j.jmb.2015.01.008. Epub 2015 Jan 23. J Mol Biol. 2015. PMID: 25623306 Free PMC article.
-
The architecture of Tetrahymena telomerase holoenzyme.Nature. 2013 Apr 11;496(7444):187-92. doi: 10.1038/nature12062. Epub 2013 Apr 3. Nature. 2013. PMID: 23552895 Free PMC article.
-
Human telomerase: biogenesis, trafficking, recruitment, and activation.Genes Dev. 2015 Jun 1;29(11):1095-105. doi: 10.1101/gad.263863.115. Genes Dev. 2015. PMID: 26063571 Free PMC article. Review.
-
Differential impact of a dyskeratosis congenita mutation in TPP1 on mouse hematopoiesis and germline.Life Sci Alliance. 2021 Oct 13;5(1):e202101208. doi: 10.26508/lsa.202101208. Print 2022 Jan. Life Sci Alliance. 2021. PMID: 34645668 Free PMC article.
-
The N Terminus of the OB Domain of Telomere Protein TPP1 Is Critical for Telomerase Action.Cell Rep. 2018 Jan 30;22(5):1132-1140. doi: 10.1016/j.celrep.2018.01.012. Cell Rep. 2018. PMID: 29386102 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

