Gonadotrope plasticity at cellular and population levels
- PMID: 22893721
- PMCID: PMC3685717
- DOI: 10.1210/en.2012-1360
Gonadotrope plasticity at cellular and population levels
Abstract
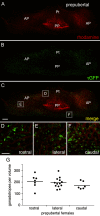
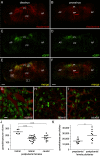
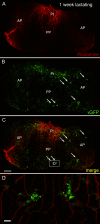
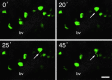
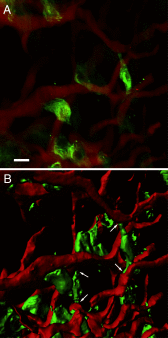
Hormone-secreting cells within the anterior pituitary gland may form organized and interdigitated networks that adapt to changing endocrine conditions in different physiological contexts. For gonadotropes, this might reflect a strategy to cope with acute changes throughout different female reproductive stages. The current study examined gonadotropes in female mice at characteristically different hormonal stages: prepubertal, postpubertal, and lactating. Gonadotrope plasticity was examined at the level of the whole population and single cells at different stages by imaging both fixed and live pituitary slices. The use of a model animal providing for the identification of selectively fluorescent gonadotropes allowed the particular advantage of defining cellular plasticity specifically for gonadotropes. In vivo analyses of gonadotropes relative to vasculature showed significantly different gonadotrope distributions across physiological states. Video microscopy studies using live slices ex vivo demonstrated pituitary cell plasticity in the form of movements and protrusions in response to GnRH. As positive feedback from rising estradiol levels is important for priming the anterior pituitary gland for the LH surge, experiments provide evidence of estradiol effects on GnRH signaling in gonadotropes. The experiments presented herein provide new insight into potential plasticity of gonadotropes within the anterior pituitary glands of female mice.
Figures
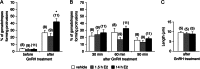
Similar articles
-
Plasticity of Anterior Pituitary Gonadotrope Cells Facilitates the Pre-Ovulatory LH Surge.Front Endocrinol (Lausanne). 2021 Feb 4;11:616053. doi: 10.3389/fendo.2020.616053. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33613451 Free PMC article. Review.
-
Enrichment of ovine gonadotropes via adenovirus gene targeting enhances assessment of transcriptional changes in response to estradiol-17 beta†.Biol Reprod. 2020 Feb 12;102(1):156-169. doi: 10.1093/biolre/ioz166. Biol Reprod. 2020. PMID: 31504222 Free PMC article.
-
Molecular Plasticity of Male and Female Murine Gonadotropes Revealed by mRNA Sequencing.Endocrinology. 2016 Mar;157(3):1082-93. doi: 10.1210/en.2015-1836. Epub 2015 Dec 17. Endocrinology. 2016. PMID: 26677881
-
Functional characterization of genetically labeled gonadotropes.Endocrinology. 2008 Jun;149(6):2701-11. doi: 10.1210/en.2007-1502. Epub 2008 Mar 6. Endocrinology. 2008. PMID: 18325995
-
Gonadotrope plasticity at cellular, population and structural levels: A comparison between fishes and mammals.Gen Comp Endocrinol. 2020 Feb 1;287:113344. doi: 10.1016/j.ygcen.2019.113344. Epub 2019 Nov 30. Gen Comp Endocrinol. 2020. PMID: 31794734 Review.
Cited by
-
S100a4-Cre-mediated deletion of Patched1 causes hypogonadotropic hypogonadism: role of pituitary hematopoietic cells in endocrine regulation.JCI Insight. 2019 Jul 2;5(14):e126325. doi: 10.1172/jci.insight.126325. JCI Insight. 2019. PMID: 31265437 Free PMC article.
-
Commentary on "Classifications of Anterior Pituitary Cell Types With Immunoenzyme Histochemistry": Dr. Paul Nakane Blazed the Trail to Modern Technology.J Histochem Cytochem. 2023 Jan;71(1):27-41. doi: 10.1369/00221554221146837. Epub 2022 Dec 21. J Histochem Cytochem. 2023. PMID: 36541702 Free PMC article. No abstract available.
-
The Neurod1/4-Ntrk3-Src pathway regulates gonadotrope cell adhesion and motility.Cell Death Discov. 2023 Sep 1;9(1):327. doi: 10.1038/s41420-023-01615-7. Cell Death Discov. 2023. PMID: 37658038 Free PMC article.
-
Pituitary stem cells: past, present and future perspectives.Nat Rev Endocrinol. 2024 Feb;20(2):77-92. doi: 10.1038/s41574-023-00922-4. Epub 2023 Dec 15. Nat Rev Endocrinol. 2024. PMID: 38102391 Free PMC article. Review.
-
Recent Advances in the Understanding of Gonadotrope Lineage Differentiation in the Developing Pituitary.Neuroendocrinology. 2025;115(2):195-210. doi: 10.1159/000542513. Epub 2024 Nov 11. Neuroendocrinology. 2025. PMID: 39527929 Free PMC article. Review.
References
-
- Itoh J, Serizawa A, Kawai K, Ishii Y, Teramoto A, Osamura RY. 2003. Vascular networks and endothelial cells in the rat experimental pituitary glands and in the human pituitary adenomas. Microsc Res Tech 60:231–235 - PubMed
-
- Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. 2005. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci USA 102:16880–16885 - PMC - PubMed
-
- Sanchez-Cardenas C, Fontanaud P, He Z, Lafont C, Meunier AC, Schaeffer M, Carmignac D, Molino F, Coutry N, Bonnefont X, Gouty-Colomer LA, Gavois E, Hodson DJ, Le Tissier P, Robinson IC, Mollard P. 2010. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci USA 107:21878–21883 - PMC - PubMed
-
- Schaeffer M, Hodson DJ, Lafont C, Mollard P. 2011. Endocrine cells and blood vessels work in tandem to generate hormone pulses. J Mol Endocrinol 47:R59–R66 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

