Comparison of usefulness of clinical diagnostic criteria for hepatocellular carcinoma in a hepatitis B endemic area
- PMID: 22893869
- PMCID: PMC3415877
- DOI: 10.3350/cmh.2012.18.2.185
Comparison of usefulness of clinical diagnostic criteria for hepatocellular carcinoma in a hepatitis B endemic area
Abstract
Background/aims: We compared the accuracy and usefulness of clinical diagnostic criteria for hepatocellular carcinoma in a hepatitis B virus (HBV)-endemic area.
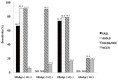
Methods: We reviewed the medical records of 355 patients who had undergone liver resection or biopsy at our institution between January 2008 and December 2009. These patients were reevaluated using four noninvasive diagnostic criteria for hepatocellular carcinoma proposed by the European Association for the Study of the Liver (EASL), the American Association for the Study of Liver Diseases (AASLD), the Korean Liver Cancer Study Group and the National Cancer Center (KLCSG/NCC), and National Comprehensive Cancer Network (NCCN) guidelines.
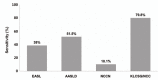
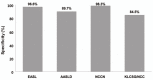
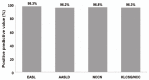
Results: The overall sensitivity was highest using the KLCSG/NCC criteria (79.8%), followed by the AASLD (51.5%), EASL (38.4%), and NCCN (10.1%; P<0.001) criteria, whereas the specificity (84.5-98.3%) and positive predictive value (96.2-98.3%) were similar for all of the criteria. The KLCSG/NCC criteria had an acceptable false-positive rate and the highest sensitivity among all of the patients, including those positive for HBsAg, those without liver cancer, and those with a tumor of at least 2 cm.
Conclusions: The KLCSG/NCC and AASLD criteria exhibited the highest sensitivity, and all four guidelines had a high specificity among all of the patients. Based on the sensitivity and false-positive rate, the KLCSG/NCC criteria was the most useful in the majority of patients. Inclusion of HBV infection in the clinical diagnostic criteria for hepatocellular carcinoma would be reasonable and may lead to an improvement in the sensitivity, with acceptable false-positive rates, in HBV-endemic areas.
Keywords: Clinical diagnostic criteria; Comparison; Hepatocellular carcinoma.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

Comment in
-
Noninvasive diagnostic criteria for hepatocellular carcinoma.Clin Mol Hepatol. 2012 Jun;18(2):174-7. doi: 10.3350/cmh.2012.18.2.174. Epub 2012 Jun 26. Clin Mol Hepatol. 2012. PMID: 22893867 Free PMC article. No abstract available.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. - PubMed
-
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. - PubMed
-
- Song IH, Kim KS. Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol. 2009;15(Suppl 6):S50–S59. - PubMed
-
- Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

