Monte Carlo simulation of a compact microbeam radiotherapy system based on carbon nanotube field emission technology
- PMID: 22894391
- PMCID: PMC3411585
- DOI: 10.1118/1.4728220
Monte Carlo simulation of a compact microbeam radiotherapy system based on carbon nanotube field emission technology
Abstract
Purpose: Microbeam radiation therapy (MRT) is an experimental radiotherapy technique that has shown potent antitumor effects with minimal damage to normal tissue in animal studies. This unique form of radiation is currently only produced in a few large synchrotron accelerator research facilities in the world. To promote widespread translational research on this promising treatment technology we have proposed and are in the initial development stages of a compact MRT system that is based on carbon nanotube field emission x-ray technology. We report on a Monte Carlo based feasibility study of the compact MRT system design.
Methods: Monte Carlo calculations were performed using EGSnrc-based codes. The proposed small animal research MRT device design includes carbon nanotube cathodes shaped to match the corresponding MRT collimator apertures, a common reflection anode with filter, and a MRT collimator. Each collimator aperture is sized to deliver a beam width ranging from 30 to 200 μm at 18.6 cm source-to-axis distance. Design parameters studied with Monte Carlo include electron energy, cathode design, anode angle, filtration, and collimator design. Calculations were performed for single and multibeam configurations.
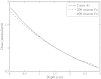
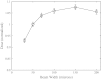
Results: Increasing the energy from 100 kVp to 160 kVp increased the photon fluence through the collimator by a factor of 1.7. Both energies produced a largely uniform fluence along the long dimension of the microbeam, with 5% decreases in intensity near the edges. The isocentric dose rate for 160 kVp was calculated to be 700 Gy∕min∕A in the center of a 3 cm diameter target. Scatter contributions resulting from collimator size were found to produce only small (<7%) changes in the dose rate for field widths greater than 50 μm. Dose vs depth was weakly dependent on filtration material. The peak-to-valley ratio varied from 10 to 100 as the separation between adjacent microbeams varies from 150 to 1000 μm.
Conclusions: Monte Carlo simulations demonstrate that the proposed compact MRT system design is capable of delivering a sufficient dose rate and peak-to-valley ratio for small animal MRT studies.
Figures










References
-
- Yu C., Shepard D., Earl M., Cao D., Luan S., Wang C., and Chen D. Z., “New developments in intensity modulated radiation therapy,” Technol. Cancer Res. Treat. 5, 451–464 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

