A quantitative study of motion estimation methods on 4D cardiac gated SPECT reconstruction
- PMID: 22894443
- PMCID: PMC3422358
- DOI: 10.1118/1.4738377
A quantitative study of motion estimation methods on 4D cardiac gated SPECT reconstruction
Abstract
Purpose: Motion-compensated temporal processing can have a major impact on improving the image quality in gated cardiac single photon emission computed tomography (SPECT). In this work, we investigate the effect of different optical flow estimation methods for motion-compensated temporal processing in gated SPECT. In particular, we explore whether better motion estimation can substantially improve reconstructed image quality, and how the estimated motion would compare to the ideal case of known motion in terms of reconstruction.
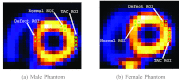
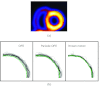
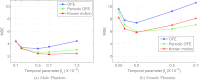
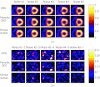
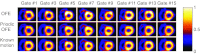
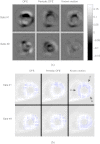
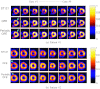
Methods: We consider the following three methods for obtaining the image motion in 4D reconstruction: (1) the Horn-Schunck optical flow equation (OFE) method, (2) a recently developed periodic OFE method, and (3) known cardiac motion derived from the NURBS-based cardiac-torso (NCAT) phantom. The periodic OFE method is used to exploit the inherent periodic nature in cardiac gated images. In this method, the optical flow in a sequence is modeled by a Fourier harmonic representation, which is then estimated from the image data. We study the impact of temporal processing on 4D reconstructions when the image motion is obtained with the different methods above. For quantitative evaluation, we use simulated imaging with multiple noise realizations from the NCAT phantom, where different patient geometry and lesion sizes are also considered. To quantify the reconstruction results, we use the following measures of reconstruction accuracy and defect detection in the myocardium: (1) overall error level in the myocardium, (2) regional accuracy of the left ventricle (LV) wall, (3) accuracy of regional time activity curves of the LV, and (4) perfusion defect detectability with a channelized Hotelling observer (CHO). In addition, we also examine the effect of noise on the distortion in the reconstructed LV wall shape by detecting its contours. As a preliminary demonstration, these methods are also tested on two sets of clinical acquisitions.
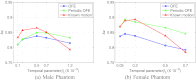
Results: For the different quantitative measures considered, the periodic OFE further improved the reconstruction accuracy of the myocardium compared to OFE in 4D reconstruction; its improvement in reconstruction almost matched that of the known motion. Specifically, the overall mean-squared error in the myocardium was reduced by over 20% with periodic OFE; with noise level fixed at 10%, the regional bias on the LV was reduced from 20% (OFE) to 14% (periodic OFE), compared to 11% by the known motion. In addition, the CHO results show that there was also improvement in lesion detectability with the periodic OFE. The regional time activity curves obtained with the periodic OFE were also observed to be more consistent with the reference; in addition, the contours of the reconstructed LV wall with the periodic OFE were demonstrated to show less degree of variations among different noise realizations. Such improvements were also consistent with the results obtained from the clinical acquisitions.
Conclusions: Use of improved optical flow estimation can further improve the accuracy of reconstructed images in 4D. The periodic OFE method not only can achieve improvements over the traditional OFE, but also can almost match that of the known motion in terms of the several quality measures considered.
Figures



References
-
- Garcia E. V., “Imaging guidelines for nuclear cardiology procedures part I,” J. Nucl. Cardiol. 3, G1–G46 (1996). - PubMed
-
- Klein G. J., Reutter B. W., and Huesman R. H., “Non-rigid summing of gated PET via optical flow,” IEEE Trans. Nucl. Sci. 44, 1509–1512 (1997). 10.1109/23.632704 - DOI
-
- Mair B. A., Gilland D. R., and Cao Z., “Simultaneous motion estimation and image reconstruction from gated data,” in Proceedings of the IEEE International Symposium on Biomedical Imaging: Macro to Nano (IEEE, 2002), pp. 661–664.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

