Adenosine A2B receptor-mediated leukemia inhibitory factor release from astrocytes protects cortical neurons against excitotoxicity
- PMID: 22894638
- PMCID: PMC3458985
- DOI: 10.1186/1742-2094-9-198
Adenosine A2B receptor-mediated leukemia inhibitory factor release from astrocytes protects cortical neurons against excitotoxicity
Abstract
Background: Neuroprotective and neurotrophic properties of leukemia inhibitory factor (LIF) have been widely reported. In the central nervous system (CNS), astrocytes are the major source for LIF, expression of which is enhanced following disturbances leading to neuronal damage. How astrocytic LIF expression is regulated, however, has remained an unanswered question. Since neuronal stress is associated with production of extracellular adenosine, we investigated whether LIF expression in astrocytes was mediated through adenosine receptor signaling.
Methods: Mouse cortical neuronal and astrocyte cultures from wild-type and adenosine A(2B) receptor knock-out animals, as well as adenosine receptor agonists/antagonists and various enzymatic inhibitors, were used to study LIF expression and release in astrocytes. When needed, a one-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test was used for statistical analysis.
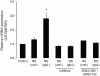
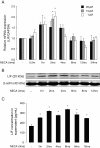
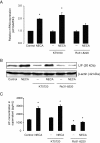
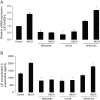
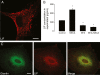
Results: We show here that glutamate-stressed cortical neurons induce LIF expression through activation of adenosine A(2B) receptor subtype in cultured astrocytes and require signaling of protein kinase C (PKC), mitogen-activated protein kinases (MAPKs: p38 and ERK1/2), and the nuclear transcription factor (NF)-κB. Moreover, LIF concentration in the supernatant in response to 5'-N-ethylcarboxamide (NECA) stimulation was directly correlated to de novo protein synthesis, suggesting that LIF release did not occur through a regulated release pathway. Immunocytochemistry experiments show that LIF-containing vesicles co-localize with clathrin and Rab11, but not with pHogrin, Chromogranin (Cg)A and CgB, suggesting that LIF might be secreted through recycling endosomes. We further show that pre-treatment with supernatants from NECA-treated astrocytes increased survival of cultured cortical neurons against glutamate, which was absent when the supernatants were pre-treated with an anti-LIF neutralizing antibody.
Conclusions: Adenosine from glutamate-stressed neurons induces rapid LIF release in astrocytes. This rapid release of LIF promotes the survival of cortical neurons against excitotoxicity.
Figures





Similar articles
-
Oncostatin M promotes excitotoxicity by inhibiting glutamate uptake in astrocytes: implications in HIV-associated neurotoxicity.J Neuroinflammation. 2016 Jun 10;13(1):144. doi: 10.1186/s12974-016-0613-8. J Neuroinflammation. 2016. PMID: 27287400 Free PMC article.
-
Activation of Bradykinin B2 Receptors in Astrocytes Stimulates the Release of Leukemia Inhibitory Factor for Autocrine and Paracrine Signaling.Int J Mol Sci. 2024 Dec 5;25(23):13079. doi: 10.3390/ijms252313079. Int J Mol Sci. 2024. PMID: 39684791 Free PMC article.
-
Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity.Neuropharmacology. 2002 Dec;43(7):1173-9. doi: 10.1016/s0028-3908(02)00217-4. Neuropharmacology. 2002. PMID: 12504924
-
ATP and repetitive electric stimulation increases leukemia inhibitory factor expression in astrocytes: A potential role for astrocytes in the action mechanism of electroconvulsive therapy.Psychiatry Clin Neurosci. 2020 May;74(5):311-317. doi: 10.1111/pcn.12986. Epub 2020 Mar 5. Psychiatry Clin Neurosci. 2020. PMID: 32022358
-
Taurine and Astrocytes: A Homeostatic and Neuroprotective Relationship.Front Mol Neurosci. 2022 Jul 5;15:937789. doi: 10.3389/fnmol.2022.937789. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35866158 Free PMC article. Review.
Cited by
-
Astrocyte-derived exosomes protect hippocampal neurons after traumatic brain injury by suppressing mitochondrial oxidative stress and apoptosis.Aging (Albany NY). 2021 Sep 13;13(17):21642-21658. doi: 10.18632/aging.203508. Epub 2021 Sep 13. Aging (Albany NY). 2021. PMID: 34516406 Free PMC article.
-
Mesenchymal Stem Cells Attenuated Blood-Brain Barrier Disruption via Downregulation of Aquaporin-4 Expression in EAE Mice.Mol Neurobiol. 2020 Sep;57(9):3891-3901. doi: 10.1007/s12035-020-01998-z. Epub 2020 Jul 1. Mol Neurobiol. 2020. PMID: 32613467 Free PMC article.
-
Glial Cells Promote Myelin Formation and Elimination.Front Cell Dev Biol. 2021 May 11;9:661486. doi: 10.3389/fcell.2021.661486. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34046407 Free PMC article. Review.
-
Adenosinergic Signaling as a Key Modulator of the Glioma Microenvironment and Reactive Astrocytes.Front Neurosci. 2022 Jan 5;15:648476. doi: 10.3389/fnins.2021.648476. eCollection 2021. Front Neurosci. 2022. PMID: 35069091 Free PMC article. Review.
-
Enteric glial adenosine 2B receptor signaling mediates persistent epithelial barrier dysfunction following acute DSS colitis.Mucosal Immunol. 2022 May;15(5):964-976. doi: 10.1038/s41385-022-00550-7. Epub 2022 Jul 22. Mucosal Immunol. 2022. PMID: 35869148 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

