Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions
- PMID: 22894755
- PMCID: PMC3472269
- DOI: 10.1186/1475-2840-11-99
Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions
Abstract
Background: Far infra-red (IFR) therapy was shown to exert beneficial effects in cardiovascular system, but effects of IFR on endothelial progenitor cell (EPC) and EPC-related vasculogenesis remain unclear. We hypothesized that IFR radiation can restore blood flow recovery in ischemic hindlimb in diabetic mice by enhancement of EPCs functions and homing process.
Materials and methods: Starting at 4 weeks after the onset of diabetes, unilateral hindlimb ischemia was induced in streptozotocin (STZ)-induced diabetic mice, which were divided into control and IFR therapy groups (n = 6 per group). The latter mice were placed in an IFR dry sauna at 34°C for 30 min once per day for 5 weeks.
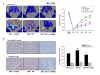
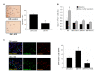
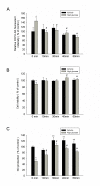
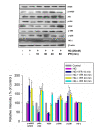
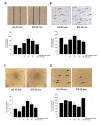
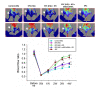
Results: Doppler perfusion imaging demonstrated that the ischemic limb/normal side blood perfusion ratio in the thermal therapy group was significantly increased beyond that in controls, and significantly greater capillary density was seen in the IFR therapy group. Flow cytometry analysis showed impaired EPCs (Sca-1(+)/Flk-1(+)) mobilization after ischemia surgery in diabetic mice with or without IFR therapy (n = 6 per group). However, as compared to those in the control group, bone marrow-derived EPCs differentiated into endothelial cells defined as GFP(+)/CD31(+) double-positive cells were significantly increased in ischemic tissue around the vessels in diabetic mice that received IFR radiation. In in-vitro studies, cultured EPCs treated with IFR radiation markedly augmented high glucose-impaired EPC functions, inhibited high glucose-induced EPC senescence and reduced H(2)O(2) production. Nude mice received human EPCs treated with IFR in high glucose medium showed a significant improvement in blood flow recovery in ischemic limb compared to those without IFR therapy. IFR therapy promoted blood flow recovery and new vessel formation in STZ-induced diabetic mice.
Conclusions: Administration of IFR therapy promoted collateral flow recovery and new vessel formation in STZ-induced diabetic mice, and these beneficial effects may derive from enhancement of EPC functions and homing process.
Figures

References
-
- Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, Hachamovitch R, Szulc M, Kligfield PD, Okin PM, Hahn RT, Devereux RB, Post MR, Hackett NR, Foster T, Grasso TM, Lesser ML, Isom OW, Crystal RG. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100(5):468–474. doi: 10.1161/01.CIR.100.5.468. - DOI - PubMed
-
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. - PMC - PubMed
-
- Adeghate E. Molecular and cellular basis of the etiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem. 2004;261(1–2):187–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

