Glutamate system, amyloid ß peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology
- PMID: 22894822
- PMCID: PMC3529221
- DOI: 10.1503/jpn.110190
Glutamate system, amyloid ß peptides and tau protein: functional interrelationships and relevance to Alzheimer disease pathology
Abstract
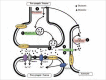
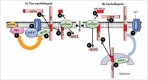
Alzheimer disease is the most prevalent form of dementia globally and is characterized premortem by a gradual memory loss and deterioration of higher cognitive functions and postmortem by neuritic plaques containing amyloid ß peptide and neurofibrillary tangles containing phospho-tau protein. Glutamate is the most abundant neurotransmitter in the brain and is essential to memory formation through processes such as long-term potentiation and so might be pivotal to Alzheimer disease progression. This review discusses how the glutamatergic system is impaired in Alzheimer disease and how interactions of amyloid ß and glutamate influence synaptic function, tau phosphorylation and neurodegeneration. Interestingly, glutamate not only influences amyloid ß production, but also amyloid ß can alter the levels of glutamate at the synapse, indicating that small changes in the concentrations of both molecules could influence Alzheimer disease progression. Finally, we describe how the glutamate receptor antagonist, memantine, has been used in the treatment of individuals with Alzheimer disease and discuss its effectiveness.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

