p53 negatively regulates Aurora A via both transcriptional and posttranslational regulation
- PMID: 22894933
- PMCID: PMC3466554
- DOI: 10.4161/cc.21732
p53 negatively regulates Aurora A via both transcriptional and posttranslational regulation
Abstract
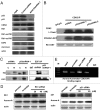
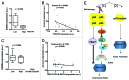
p53 plays an important role in mitotic checkpoint, but what its role is remains enigmatic. Aurora A is a Ser/Thr kinase involved in correcting progression of mitosis. Here, we show that p53 is a negative regulator for Aurora A. We found that p53 deficiency leads to Aurora A elevation. Ectopic expression of p53 or DNA damage-induced expression of p53 can suppress the expression of Aurora A. Mechanistic studies show that p53 is a negative regulator for Aurora A expression through both transcriptional and posttranslational regulation. p53 knockdown in cancer cells reduces the level of p21, which, in turn, increases the activity of CDK2 followed by induction of Rb1 hyperphosphorylation and its dissociation with transcriptional factor E2F3. E2F3 can bind to Aurora A gene promoter, potentiating Aurora A gene expression and p53 deficiency, enhancing the binding of E2F3 on Aurora A promoter. Also, p53 deficiency leads to decelerating Aurora A's turnover rate, due to the fact that p53 deficiency causes the downregulation of Fbw7α, a component of E3 ligase of Aurora A. Consistently, p53 knockdown-mediated Aurora A elevation is mitigated when Fbw7α is ectopically expressed. Thus, p53-mediated Aurora A degradation requires Fbw7α expression. Significantly, inverse correlation between p53 and Aurora A elevation is translated into the deregulation of centrosome amplification. p53 knockdown leads to high percentages of cells with abnormal amplification of centrosome. These data suggest that p53 is an important negative regulator of Aurora A, and that loss of p53 in many types of cancer could lead to abnormal elevation of Aurora A and dysregulated mitosis, which provides a growth advantage for cancer cells.
Figures




Comment in
-
p53-Aurora A mitotic feedback loop regulates cell cycle progression and genomic stability.Cell Cycle. 2012 Oct 15;11(20):3719-20. doi: 10.4161/cc.22113. Epub 2012 Sep 14. Cell Cycle. 2012. PMID: 22982999 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
