Lithocholic acid extends longevity of chronologically aging yeast only if added at certain critical periods of their lifespan
- PMID: 22894934
- PMCID: PMC3466555
- DOI: 10.4161/cc.21754
Lithocholic acid extends longevity of chronologically aging yeast only if added at certain critical periods of their lifespan
Abstract
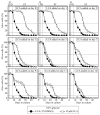
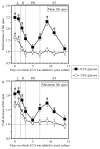
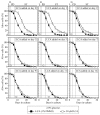
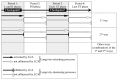
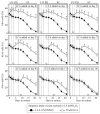
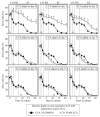
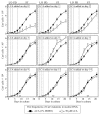
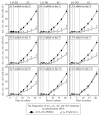
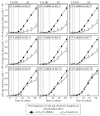
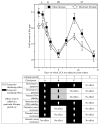
Our studies revealed that LCA (lithocholic bile acid) extends yeast chronological lifespan if added to growth medium at the time of cell inoculation. We also demonstrated that longevity in chronologically aging yeast is programmed by the level of metabolic capacity and organelle organization that they developed before entering a quiescent state and, thus, that chronological aging in yeast is likely to be the final step of a developmental program progressing through at least one checkpoint prior to entry into quiescence. Here, we investigate how LCA influences longevity and several longevity-defining cellular processes in chronologically aging yeast if added to growth medium at different periods of the lifespan. We found that LCA can extend longevity of yeast under CR (caloric restriction) conditions only if added at either of two lifespan periods. One of them includes logarithmic and diauxic growth phases, whereas the other period exists in early stationary phase. Our findings suggest a mechanism linking the ability of LCA to increase the lifespan of CR yeast only if added at either of the two periods to its differential effects on various longevity-defining processes. In this mechanism, LCA controls these processes at three checkpoints that exist in logarithmic/diauxic, post-diauxic and early stationary phases. We therefore hypothesize that a biomolecular longevity network progresses through a series of checkpoints, at each of which (1) genetic, dietary and pharmacological anti-aging interventions modulate a distinct set of longevity-defining processes comprising the network; and (2) checkpoint-specific master regulators monitor and govern the functional states of these processes.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
