Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding
- PMID: 22894939
- PMCID: PMC3441110
- DOI: 10.3945/ajcn.112.039503
Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding
Abstract
Background: The inefficiency of HIV breast-milk transmission may be caused by the presence of immunologically active factors, including human milk oligosaccharides (HMOs).
Objective: We investigated whether HMO concentrations are associated with a reduced risk of postnatal HIV transmission.
Design: A nested case-control study was conducted within a larger cohort study of HIV-infected women and their infants followed from birth to 24 mo in Lusaka, Zambia. Breast-milk samples collected at 1 mo from 81 HIV-infected women who transmitted via breastfeeding, a random sample of 86 HIV-infected women who did not transmit despite breastfeeding, and 36 uninfected breastfeeding women were selected. Total and specific HMO concentrations were measured by HPLC and compared between groups with adjustment for confounders by using logistic regression.
Results: HIV-infected women with total HMOs above the median (1.87 g/L) were less likely to transmit via breastfeeding (OR: 0.45; 95% CI: 0.21, 0.97; P = 0.04) after adjustment for CD4 count and breast-milk HIV RNA concentrations; a trend toward higher concentrations of lacto-N-neotetraose being associated with reduced transmission (OR: 0.49; 95% CI: 0.23, 1.04; P = 0.06) was also observed. The proportion of 3'-sialyllactose (3'-SL) per total HMOs was higher among transmitting than among nontransmitting women (P = 0.003) and correlated with higher plasma and breast-milk HIV RNA and lower CD4 counts. Neither Secretor nor Lewis status distinguished between transmitting and nontransmitting women.
Conclusions: Higher concentrations of non-3'-SL HMOs were associated with protection against postnatal HIV transmission independent of other known risk factors. Further study of these novel, potentially anti-HIV components of breast milk is warranted.
Trial registration: ClinicalTrials.gov NCT00310726.
Figures
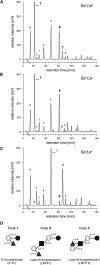

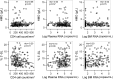
References
-
- Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Msellati P, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis 2004;189:2154–66 - PubMed
-
- Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol 2004;4:565–72 - PubMed
-
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 2000;20:699–722 - PubMed
-
- Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr 2006;136:2127–30 - PubMed
-
- Bode L. Human milk oligosaccharides: prebiotics and beyond. Nutr Rev 2009;67(suppl 2):S183–91 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

