Comparison of complement dependent lytic, hemagglutination inhibition and microneutralization antibody responses in influenza vaccinated individuals
- PMID: 22894961
- PMCID: PMC3579901
- DOI: 10.4161/hv.21025
Comparison of complement dependent lytic, hemagglutination inhibition and microneutralization antibody responses in influenza vaccinated individuals
Abstract
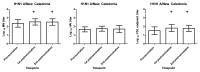
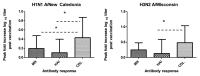
Virus specific, non-neutralizing antibodies such as complement dependent lytic (CDL) antibodies may reduce morbidity following infection through the clearance of infectious virus particles and infected cells. We examined hemagglutination inhibition (HAI), microneutralization (MN) and CDL antibody titers to influenza A H1 and H3 virus strains in 23 healthy young adults who received the 2005-2006 trivalent inactivated influenza vaccine. Post vaccination, we detected statistically significant increases in MN and CDL antibodies but not in HAI antibodies. Statistically significantly higher fold increases in CDL antibodies post vaccination were seen compared with MN and HAI antibodies post vaccination. However, the overall fold increases were modest, likely related to the fact that most of the subjects had received influenza vaccination previously. This study showed that influenza vaccination is not only capable of increasing the level of antibodies that neutralize virus but also antibodies that can cause lysis of infected cells. The biological significance of these CDL antibodies merits further investigation in clinical studies.
Figures

References
-
- de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. - PubMed
-
- Szretter KJ, Balish AL, Katz JM. Influenza: propagation, quantification, and storage. Curr Protoc Microbiol 2006; Chapter 15:Unit 15G 1. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
