The potential bioproduction of the pharmaceutical agent sakuranetin, a flavonoid phytoalexin in rice
- PMID: 22895058
- PMCID: PMC3489713
- DOI: 10.4161/bioe.21546
The potential bioproduction of the pharmaceutical agent sakuranetin, a flavonoid phytoalexin in rice
Abstract
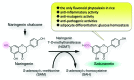
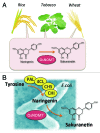
Sakuranetin, the major flavonoid phytoalexin in rice, can be induced by ultraviolet (UV) irradiation, treatment with CuCl 2 or jasmonic acid (JA), or phytopathogenic infection. In addition to sakuranetin's biological significance on disease resistance in rice, its broad bioactivities have recently been described. Results from these studies have shown that sakuranetin is a useful compound as a plant antibiotic and a potential pharmaceutical agent. Sakuranetin is biosynthesized from naringenin, a precursor of sakuranetin, by naringenin 7-O-methyltransferase (NOMT), but the relevant gene has not yet been identified in rice. Recently, we identified the OsNOMT gene, which is involved in the final step of sakuranetin biosynthesis in rice. In previous studies, OsNOMT was purified to apparent homogeneity from UV-treated wild-type rice leaves; however, the purified protein, termed OsCOMT1, exhibited caffeic acid 3-O-methyltransferase (COMT) activity, but not NOMT activity. Based on the analysis of an oscomt1 T-DNA tagged mutant, we determined that OsCOMT1 did not contribute to sakuranetin production in rice in vivo. Therefore, we took advantage of the oscomt1 mutant to purify OsNOMT. A crude protein preparation from UV-treated oscomt1 leaves was subjected to three sequential purification steps resulting in a 400-fold purification from the crude enzyme preparation with a minor band at an apparent molecular mass of 40 kDa in the purest enzyme preparation. Matrix-assisted laser desorption/ionization time of flight/time of flight analysis showed that the 40 kDa protein band included two O-methyltransferase-like proteins, but one of the proteins encoded by Os12g0240900 exhibited clear NOMT activity; thus, this gene was designated OsNOMT. Gene expression was induced by treatment with jasmonic acid in rice leaves prior to sakuranetin accumulation, and the recombinant protein showed reasonable kinetic properties to NOMT. Identification of the OsNOMT gene enables the production of large amounts of sakuranetin through transgenic rice and microorganisms. This finding also allows for the generation of disease-resistant and sakuranetin biofortified rice in the future.
Figures

Similar articles
-
Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice.J Biol Chem. 2012 Jun 1;287(23):19315-25. doi: 10.1074/jbc.M112.351270. Epub 2012 Apr 9. J Biol Chem. 2012. PMID: 22493492 Free PMC article.
-
Natural variation in the expression and catalytic activity of a naringenin 7-O-methyltransferase influences antifungal defenses in diverse rice cultivars.Plant J. 2020 Mar;101(5):1103-1117. doi: 10.1111/tpj.14577. Epub 2019 Nov 18. Plant J. 2020. PMID: 31630460
-
OsMYC2, an essential factor for JA-inductive sakuranetin production in rice, interacts with MYC2-like proteins that enhance its transactivation ability.Sci Rep. 2017 Jan 9;7:40175. doi: 10.1038/srep40175. Sci Rep. 2017. PMID: 28067270 Free PMC article.
-
Sakuranetin and its therapeutic potentials - a comprehensive review.Z Naturforsch C J Biosci. 2022 Jul 13;78(1-2):27-48. doi: 10.1515/znc-2022-0024. Print 2023 Jan 27. Z Naturforsch C J Biosci. 2022. PMID: 35844107 Review.
-
A Review on Sources and Pharmacological Aspects of Sakuranetin.Nutrients. 2020 Feb 18;12(2):513. doi: 10.3390/nu12020513. Nutrients. 2020. PMID: 32085443 Free PMC article. Review.
Cited by
-
Computational Study of Asian Propolis Compounds as Potential Anti-Type 2 Diabetes Mellitus Agents by Using Inverse Virtual Screening with the DIA-DB Web Server, Tanimoto Similarity Analysis, and Molecular Dynamic Simulation.Molecules. 2022 Jun 21;27(13):3972. doi: 10.3390/molecules27133972. Molecules. 2022. PMID: 35807241 Free PMC article.
-
Biofortified Rice Provides Rich Sakuranetin in Endosperm.Rice (N Y). 2024 Mar 2;17(1):19. doi: 10.1186/s12284-024-00697-w. Rice (N Y). 2024. PMID: 38430431 Free PMC article.
-
Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study.PLoS One. 2017 Jan 19;12(1):e0170077. doi: 10.1371/journal.pone.0170077. eCollection 2017. PLoS One. 2017. PMID: 28103258 Free PMC article.
References
-
- Koga J, Shimura M, Oshima K, Ogawa N, Yamauchi T, Ogasawara N, et al. B, C, and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron. 1995;51:7907–18. doi: 10.1016/0040-4020(95)00423-6. - DOI
-
- Koga J, Ogawa N, Yamauchi T, Kikuchi M, Ogasawara N, Shimura M. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry. 1997;44:249–53. doi: 10.1016/S0031-9422(96)00534-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
