Aurora kinase inhibitor VE 465 synergistically enhances cytotoxicity of carboplatin in ovarian cancer cells through induction of apoptosis and downregulation of histone 3
- PMID: 22895067
- PMCID: PMC3461810
- DOI: 10.4161/cbt.21045
Aurora kinase inhibitor VE 465 synergistically enhances cytotoxicity of carboplatin in ovarian cancer cells through induction of apoptosis and downregulation of histone 3
Abstract
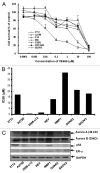
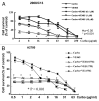
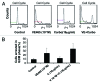
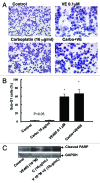
Aurora kinases are essential for regulation of chromosome segregation and cytokinesis during mitosis and play a role in growth and progression of human tumors, including ovarian cancer. Aurora A and Aurora B are frequently overexpressed in high-grade and low-grade ovarian cancers. Targeting Aurora kinases has great potential for improving the efficacy of chemotherapies of ovarian cancer. In this study, we investigated whether the Aurora kinase inhibitor, VE 465, can enhance the anti-tumor activity of carboplatin in human ovarian cancer cells. The antitumor activity of VE 465 was tested by MTT proliferative assay in multiple established human epithelial ovarian cancer cell lines of varying p53 status. VE 465 and carboplatin had a synergistic effect on cell viability in both platinum-sensitive and -resistant ovarian cancers. The growth-inhibitory effect was accompanied by reduction in expression of histone 3 and an increase in apoptosis. We conclude that VE 465 enhances the efficacy of carboplatin agents in ovarian carcinoma.
Figures


References
-
- Kavanagh JJSS, Pecoreli S, Dipertrillo A, Einhorn N. Chemotherapy in advanced ovarian cancer. Cambrige, MA: Blackwell Scientific Publications, 1998.
-
- Verschraegen CFDK, Johnes LA. Biology of gynecologic cancers. Cambrige, MA: Blackwell Scientific Publications, 1998.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
