Identification of inositol polyphosphate 4-phosphatase type II as a novel tumor resistance biomarker in human laryngeal cancer HEp-2 cells
- PMID: 22895072
- PMCID: PMC3493439
- DOI: 10.4161/cbt.21788
Identification of inositol polyphosphate 4-phosphatase type II as a novel tumor resistance biomarker in human laryngeal cancer HEp-2 cells
Abstract
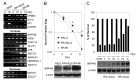
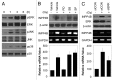
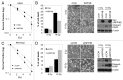
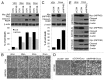
Although tumor resistance remains a significant impediment to successful radiotherapy, associated regulatory markers and detailed molecular mechanisms underlying this phenomenon are not well defined. In this study, we identified inositol polyphosphate 4-phosphatase type II (INPP4B) as a novel marker of radioresistance by systematically analyzing Unigene libraries of laryngeal cancer. INPP4B was highly expressed in radioresistant laryngeal cancer cells and was induced by treatment with either radiation or anticancer drugs in various types of cancer cells. Ectopic INPP4B overexpression increased radioresistance and anticancer drug resistance by suppressing apoptosis in HEp-2 cells. Conversely, INPP4B depletion with small interfering RNA resensitized HEp-2 as well as A549 and H1299 cells to radiation- and anticancer drug-induced apoptosis. Furthermore, radiation-induced INPP4B expression was blocked by inhibition of extracellular signal-regulated kinase (ERK). INPP4B depletion significantly attenuated radiation-induced increases in Akt phosphorylation, indicating an association of INPP4B-mediated radioresistance with Akt survival signaling. Taken together, our data suggest that ERK-dependent induction of INPP4B triggers the development of a tumor-resistance phenotype via Akt signaling and identify INPP4B as a potentially important target molecule for resolving the radioresistance of cancer cells.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
