Oridonin in combination with imatinib exerts synergetic anti-leukemia effect in Ph+ acute lymphoblastic leukemia cells in vitro by inhibiting activation of LYN/mTOR signaling pathway
- PMID: 22895079
- PMCID: PMC3493431
- DOI: 10.4161/cbt.21460
Oridonin in combination with imatinib exerts synergetic anti-leukemia effect in Ph+ acute lymphoblastic leukemia cells in vitro by inhibiting activation of LYN/mTOR signaling pathway
Abstract
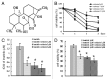
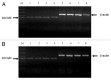
Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) is triggered by constitutively activated BCR-ABL and SRC family tyrosine kinases.They account for the activations of multiple growth-signaling pathways, including Raf/MEK/ERK, Akt/mTOR and STAT5 pathways. The BCR-ABL tyrosine kinase inhibitor imatinib is the standard treatment for Ph+ leukemia and plays efficacious role in CML. However, imatinib has few inhibitory effects on SRC tyrosine kinase with response rate of Ph+ ALL lower and relapse more frequent and quicker compared with CML. Previous studies showed that oridonin inhibits proliferation and induces apoptosis in many tumor cells. However, the anticancer activity and mechanism of oridonin in Ph+ ALL is unknown. To investigate the anticancer activity of oridonin, we examined its role in constitutively activated Akt/mTOR, Raf/MEK/ERK, STAT5 and SRC pathway, mRNA level of bcr/abl gene, cell viability and apoptosis in Ph+ ALL SUP-B15 cells. Furthermore, we detected synergetic effect of oridonin plus imatinib. Our results showed that oridonin inhibiting activations of LYN (one of SRC family kinases) and ABL and their downstream Akt/mTOR, Raf/MEK/ERK and STAT5 pathways, downregulated Bcl-2 but upregulated Bax protein and then induced apoptosis in Ph+ ALL cells. Oridonin plus imatinib exerted synergetic effects by overcoming imatinib defect of upregulating Akt/mTOR and LYN signaling. Additionally, we examined the effect of oridonin on the signaling pathways in the primary specimens from Ph+ ALL patients. Our data showed that oridonin remarkably suppressed activations of Akt/mTOR, Raf/MEK and STAT5 pathway in these primary specimens and oridonin with imatinib exerted synergetic suppressive effects on mTOR, STAT5 and LYN signaling in one imatinib resistant patient specimen. Additional evaluation of oridonin as a potential therapeutic agent for Ph+ ALL seems warranted.
Figures





References
-
- Laurent E, Talpaz M, Kantarjian H, Kurzrock R. The BCR gene and philadelphia chromosome-positive leukemogenesis. Cancer Res. 2001;61:2343–55. - PubMed
-
- Kurzrock R, Kantarjian HM, Druker BJ, Talpaz M. Philadelphia chromosome-positive leukemias: from basic mechanisms to molecular therapeutics. Ann Intern Med. 2003;138:819–30. - PubMed
-
- Cobaleda C, Gutiérrez-Cianca N, Pérez-Losada J, Flores T, García-Sanz R, González M, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human philadelphia-positive acute lymphoblastic leukemia. Blood. 2000;95:1007–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
