Oxydative stress alters nuclear shape through lamins dysregulation: a route to senescence
- PMID: 22895091
- PMCID: PMC3474660
- DOI: 10.4161/nucl.21674
Oxydative stress alters nuclear shape through lamins dysregulation: a route to senescence
Abstract
Progeroid phenotypes are mainly encountered in 2 types of syndromes: in laminopathies, which are characterized by nuclear shape abnormalities due to lamin A alteration, and in DNA damage response defect syndromes. Because lamin A dysregulation leads to DNA damages, it has been proposed that senescence occurs in both types of syndromes through the accumulation of damages. We recently showed that elevated oxidative stress is responsible for lamin B1 accumulation, nuclear shape alteration and senescence in the DDR syndrome, ataxia telangiectasia (A-T). Interestingly, overexpression of lamin B1 in wild type cells is sufficient to induce senescence without the induction of DNA damages. Here, we will discuss the importance of controlling the lamins level in order for maintenance nuclear architecture and we will comment the relationships of lamins with other senescence mechanisms. Finally, we will describe emerging data reporting redox control by lamins, leading us to propose a general mechanism by which reactive oxygen species can induce senescence through lamin dysregulation and NSA.
Figures

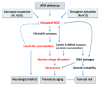
Comment on
- Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, et al. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31:1080–94. doi: 10.1038/emboj.2011.492.
Similar articles
-
Lamin B1 loss is a senescence-associated biomarker.Mol Biol Cell. 2012 Jun;23(11):2066-75. doi: 10.1091/mbc.E11-10-0884. Epub 2012 Apr 11. Mol Biol Cell. 2012. PMID: 22496421 Free PMC article.
-
Hutchinson-Gilford progeria syndrome through the lens of transcription.Aging Cell. 2013 Aug;12(4):533-43. doi: 10.1111/acel.12070. Epub 2013 Apr 19. Aging Cell. 2013. PMID: 23496208 Review.
-
The telomeric protein AKTIP interacts with A- and B-type lamins and is involved in regulation of cellular senescence.Open Biol. 2016 Aug;6(8):160103. doi: 10.1098/rsob.160103. Open Biol. 2016. PMID: 27512140 Free PMC article.
-
Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation.EMBO J. 2012 Mar 7;31(5):1080-94. doi: 10.1038/emboj.2011.492. Epub 2012 Jan 13. EMBO J. 2012. PMID: 22246186 Free PMC article.
-
Nuclear matrix, nuclear envelope and premature aging syndromes in a translational research perspective.Semin Cell Dev Biol. 2014 May;29:125-47. doi: 10.1016/j.semcdb.2014.03.021. Epub 2014 Mar 22. Semin Cell Dev Biol. 2014. PMID: 24662892 Review.
Cited by
-
iTRAQ-based analysis of progerin expression reveals mitochondrial dysfunction, reactive oxygen species accumulation and altered proteostasis.Stem Cell Res Ther. 2015 Jun 12;6(1):119. doi: 10.1186/s13287-015-0110-5. Stem Cell Res Ther. 2015. PMID: 26066325 Free PMC article.
-
Independent Mechanisms Lead to Genomic Instability in Hodgkin Lymphoma: Microsatellite or Chromosomal Instability †.Cancers (Basel). 2018 Jul 13;10(7):233. doi: 10.3390/cancers10070233. Cancers (Basel). 2018. PMID: 30011886 Free PMC article.
-
The three-dimensional organization of the genome in cellular senescence and age-associated diseases.Semin Cell Dev Biol. 2019 Jun;90:154-160. doi: 10.1016/j.semcdb.2018.07.022. Epub 2018 Jul 27. Semin Cell Dev Biol. 2019. PMID: 30031215 Free PMC article. Review.
-
ABCC6 knockdown in HepG2 cells induces a senescent-like cell phenotype.Cell Mol Biol Lett. 2017 Apr 4;22:7. doi: 10.1186/s11658-017-0036-2. eCollection 2017. Cell Mol Biol Lett. 2017. PMID: 28536638 Free PMC article.
-
Lamin B is a target for selective nuclear PQC by BAG3: implication for nuclear envelopathies.Cell Death Dis. 2019 Jan 10;10(1):23. doi: 10.1038/s41419-018-1255-9. Cell Death Dis. 2019. PMID: 30631036 Free PMC article.
References
-
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
