Blocking farnesylation of the prelamin A variant in Hutchinson-Gilford progeria syndrome alters the distribution of A-type lamins
- PMID: 22895092
- PMCID: PMC3474666
- DOI: 10.4161/nucl.21675
Blocking farnesylation of the prelamin A variant in Hutchinson-Gilford progeria syndrome alters the distribution of A-type lamins
Abstract
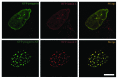
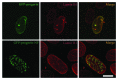
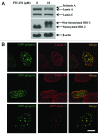
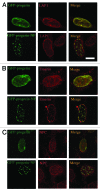
Mutations in the lamin A/C gene that cause Hutchinson-Gilford progeria syndrome lead to expression of a truncated, permanently farnesylated prelamin A variant called progerin. Blocking farnesylation leads to an improvement in the abnormal nuclear morphology observed in cells expressing progerin, which is associated with a re-localization of the variant protein from the nuclear envelope to the nuclear interior. We now show that a progerin construct that cannot be farnesylated is localized primarily in intranuclear foci and that its diffusional mobility is significantly greater than that of farnesylated progerin localized predominantly at the nuclear envelope. Expression of non-farnesylated progerin in transfected cells leads to a redistribution of lamin A and lamin C away from the nuclear envelope into intranuclear foci but does not significantly affect the localization of endogenous lamin B1 at nuclear envelope. There is a similar redistribution of lamin A and lamin C into intranuclear foci in transfected cells expressing progerin in which protein farnesylation is blocked by treatment with a protein farnesyltransferase inhibitor. Blocking farnesylation of progerin can lead to a redistribution of normal A-type lamins away from the inner nuclear envelope. This may have implications for using drugs that block protein prenylation to treat children with Hutchinson-Gilford progeria syndrome. These findings also provide additional evidence that A-type and B-type lamins can form separate microdomains within the nucleus.
Figures



Similar articles
-
Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome.Proc Natl Acad Sci U S A. 2005 Sep 6;102(36):12879-84. doi: 10.1073/pnas.0506001102. Epub 2005 Aug 29. Proc Natl Acad Sci U S A. 2005. PMID: 16129833 Free PMC article.
-
Blocking protein farnesylation improves nuclear shape abnormalities in keratinocytes of mice expressing the prelamin A variant in Hutchinson-Gilford progeria syndrome.Nucleus. 2010 Sep-Oct;1(5):432-9. doi: 10.4161/nucl.1.5.12972. Nucleus. 2010. PMID: 21326826 Free PMC article.
-
Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation.Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10291-6. doi: 10.1073/pnas.0504641102. Epub 2005 Jul 12. Proc Natl Acad Sci U S A. 2005. PMID: 16014412 Free PMC article.
-
Prelamin A farnesylation and progeroid syndromes.J Biol Chem. 2006 Dec 29;281(52):39741-5. doi: 10.1074/jbc.R600033200. Epub 2006 Nov 7. J Biol Chem. 2006. PMID: 17090536 Review.
-
Lamin A, farnesylation and aging.Exp Cell Res. 2012 Jan 1;318(1):1-7. doi: 10.1016/j.yexcr.2011.08.009. Epub 2011 Aug 16. Exp Cell Res. 2012. PMID: 21871450 Free PMC article. Review.
Cited by
-
Impact of Combined Baricitinib and FTI Treatment on Adipogenesis in Hutchinson-Gilford Progeria Syndrome and Other Lipodystrophic Laminopathies.Cells. 2023 May 9;12(10):1350. doi: 10.3390/cells12101350. Cells. 2023. PMID: 37408186 Free PMC article.
-
Chemical inhibition of NAT10 corrects defects of laminopathic cells.Science. 2014 May 2;344(6183):527-32. doi: 10.1126/science.1252651. Science. 2014. PMID: 24786082 Free PMC article.
-
Prelamin A impairs 53BP1 nuclear entry by mislocalizing NUP153 and disrupting the Ran gradient.Aging Cell. 2016 Dec;15(6):1039-1050. doi: 10.1111/acel.12506. Epub 2016 Jul 27. Aging Cell. 2016. PMID: 27464478 Free PMC article.
-
Disruption of lamin B1 and lamin B2 processing and localization by farnesyltransferase inhibitors.Nucleus. 2013 Mar-Apr;4(2):142-50. doi: 10.4161/nucl.24089. Epub 2013 Mar 1. Nucleus. 2013. PMID: 23475125 Free PMC article.
-
Gene expression profiling of fibroblasts in a family with LMNA-related cardiomyopathy reveals molecular pathways implicated in disease pathogenesis.BMC Med Genet. 2020 Jul 22;21(1):152. doi: 10.1186/s12881-020-01088-w. BMC Med Genet. 2020. PMID: 32698886 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
