Long-term propranolol use in severely burned pediatric patients: a randomized controlled study
- PMID: 22895351
- PMCID: PMC3505887
- DOI: 10.1097/SLA.0b013e318265427e
Long-term propranolol use in severely burned pediatric patients: a randomized controlled study
Abstract
Objective: To determine the safety and efficacy of propranolol given for 1 year on cardiac function, resting energy expenditure, and body composition in a prospective, randomized, single-center, controlled study in pediatric patients with large burns.
Background: Severe burns trigger a hypermetabolic response that persists for up to 2 years postburn. Propranolol given for 1 month postburn blunts this response. Whether propranolol administration for 1 year after injury provides a continued benefit is currently unclear.
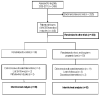
Methods: One-hundred seventy-nine pediatric patients with more than 30% total body surface area burns were randomized to control (n = 89) or 4 mg/kg/d propranolol (n = 90) for 12 months postburn. Changes in resting energy expenditure, cardiac function, and body composition were measured acutely at 3, 6, 9, and 12 months postburn. Statistical analyses included techniques that adjusted for non-normality, repeated-measures, and regression analyses. P < 0.05 was considered significant.
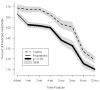
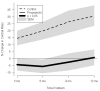
Results: Long-term propranolol treatment significantly reduced the percentage of the predicted heart rate and percentage of the predicted resting energy expenditure, decreased accumulation of central mass and central fat, prevented bone loss, and improved lean body mass accretion. There were very few adverse effects from the dose of propranolol used.
Conclusions: Propranolol treatment for 12 months after thermal injury, ameliorates the hyperdynamic, hypermetabolic, hypercatabolic, and osteopenic responses in pediatric patients. This study is registered at clinicaltrials.gov: NCT00675714.
Figures






References
-
- Pereira CT, Murphy KD, Herndon DN. Altering metabolism. J Burn Care Rehabil. 2005;26(3):194–9. - PubMed
-
- Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–902. - PubMed
-
- Wilmore DW, Aulick LH. Metabolic changes in burned patients. Surg Clin North Am. 1978;58(6):1173–87. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 HD049471/HD/NICHD NIH HHS/United States
- R01-GM56687-11S1/GM/NIGMS NIH HHS/United States
- T32-GM8256/GM/NIGMS NIH HHS/United States
- KL2RR029875/RR/NCRR NIH HHS/United States
- UL1 TR000071/TR/NCATS NIH HHS/United States
- H133A070026/PHS HHS/United States
- R01 GM056687/GM/NIGMS NIH HHS/United States
- P50 GM060338/GM/NIGMS NIH HHS/United States
- KL2 RR029875/RR/NCRR NIH HHS/United States
- P50-GM60338/GM/NIGMS NIH HHS/United States
- T32 GM008256/GM/NIGMS NIH HHS/United States
- H133A70019/PHS HHS/United States
- UL1 RR029876/RR/NCRR NIH HHS/United States
- R01-HD049471/HD/NICHD NIH HHS/United States
- UL1RR029876/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

