Diacylglycerol kinase regulates tyrosinase expression and function in human melanocytes
- PMID: 22895365
- PMCID: PMC3502659
- DOI: 10.1038/jid.2012.261
Diacylglycerol kinase regulates tyrosinase expression and function in human melanocytes
Abstract
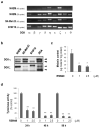
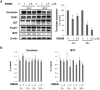
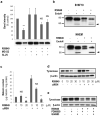
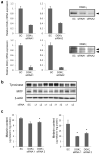
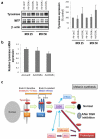
Diacylglycerol (DAG) increases the melanin content of human melanocytes in vitro and increases the pigmentation of guinea pig skin in vivo, but the mechanism(s) underlying those effects remain unknown. In this study, we characterized the role of diacylglycerol kinase (DGK), which phosphorylates DAG to generate phosphatidic acid, in the regulation of pigmentation. Ten isoforms of DGK have been identified, and we show that DGKζ is the most abundant isoform expressed by human melanocytic cells. Melanin content, tyrosinase activity, and tyrosinase protein levels were significantly reduced by a DGK inhibitor, but tyrosinase and microphthalmia-associated transcription factor messenger RNA (mRNA) levels were not changed by that inhibition, and there were no effects on the expression of other melanogenesis-related proteins. Isoform-specific small interfering RNAs showed that knockdown of DGKζ decreased melanin content and tyrosinase expression in melanocytic cells. Overexpression of DGKζ increased tyrosinase protein levels, but did not increase tyrosinase mRNA levels. Glycosidase digestion revealed that inhibition of DGK reduced only the mature form of tyrosinase, and the decrease of tyrosinase resulting from DGK inhibition could be blocked partially by protease inhibitors. These results suggest that DGK regulates melanogenesis via modulation of the posttranslational processing of tyrosinase, which may be related with the protein degradation machinery.
Figures
Comment in
-
Tyrosinase: a central regulatory protein for cutaneous pigmentation.J Invest Dermatol. 2012 Dec;132(12):2678-80. doi: 10.1038/jid.2012.324. J Invest Dermatol. 2012. PMID: 23187110
References
-
- Allan AE, Archambault M, Messana E, et al. Topically applied diacylglycerols increase pigmentation in guinea pig skin. J Invest Dermatol. 1995;105:687–692. - PubMed
-
- Ando H, Kondoh H, Ichihashi M, et al. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007;127:751–761. - PubMed
-
- Ando H, Watabe H, Valencia JC, et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase - a new aspect of ubiquitin-proteasome function. J Biol Chem. 2004;279:15427–15433. - PubMed
-
- Avila-Flores A, Santos T, Rincon E, et al. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J Biol Chem. 2005;280:10091–10099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

