State-dependent modulation of breathing in urethane-anesthetized rats
- PMID: 22895710
- PMCID: PMC6621193
- DOI: 10.1523/JNEUROSCI.0948-12.2012
State-dependent modulation of breathing in urethane-anesthetized rats
Abstract
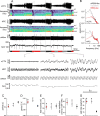
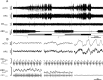
Respiratory activity is most fragile during sleep, in particular during paradoxical [or rapid eye movement (REM)] sleep and sleep state transitions. Rats are commonly used to study respiratory neuromodulation, but rodent sleep is characterized by a highly fragmented sleep pattern, thus making it very challenging to examine different sleep states and potential pharmacological manipulations within them. Sleep-like brain-state alternations occur in rats under urethane anesthesia and may be an effective and efficient model for sleep itself. The present study assessed state-dependent changes in breathing and respiratory muscle modulation under urethane anesthesia to determine their similarity to those occurring during natural sleep. Rats were anesthetized with urethane and respiratory airflow, as well as electromyographic activity in respiratory muscles were recorded in combination with local field potentials in neocortex and hippocampus to determine how breathing pattern and muscle activity are modulated with brain state. Measurements were made in normoxic, hypoxic, and hypercapnic conditions. Results were compared with recordings made from rats during natural sleep. Brain-state alternations under urethane anesthesia were closely correlated with changes in breathing rate and variability and with modulation of respiratory muscle tone. These changes closely mimicked those observed in natural sleep. Of great interest was that, during both REM and REM-like states, genioglossus muscle activity was strongly depressed and abdominal muscle activity showed potent expiratory modulation. We demonstrate that, in urethane-anesthetized rats, respiratory airflow and muscle activity are closely correlated with brain-state transitions and parallel those shown in natural sleep, providing a useful model to systematically study sleep-related changes in respiratory control.
Figures




References
-
- Antkowiak B. How do general anaesthetics work? Naturwissenschaften. 2001;88:201–213. - PubMed
-
- Aserinsky E. Periodic respiratory pattern occurring in conjunction with eye movements during sleep. Science. 1965;150:763–766. - PubMed
-
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. - PubMed
-
- Baldwin DN, Suki B, Pillow JJ, Roiha HL, Minocchieri S, Frey U. Effect of sighs on breathing memory and dynamics in healthy infants. J Appl Physiol. 2004;97:1830–1839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
