The role of cAMP response element-binding protein in estrogen negative feedback control of gonadotropin-releasing hormone neurons
- PMID: 22895714
- PMCID: PMC6621199
- DOI: 10.1523/JNEUROSCI.1333-12.2012
The role of cAMP response element-binding protein in estrogen negative feedback control of gonadotropin-releasing hormone neurons
Abstract
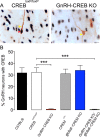
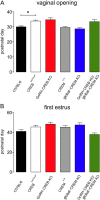
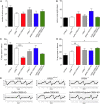
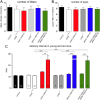
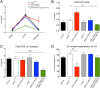
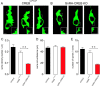
The mechanisms through which estradiol (E2) regulates gonadotropin-releasing hormone (GnRH) neurons to control fertility are unclear. Previous studies have demonstrated that E2 rapidly phosphorylates cAMP response element-binding protein (CREB) in GnRH neurons in vivo. In the present study, we used GnRH neuron-specific CREB-deleted mutant mice [GnRH-CREB knock-outs (KOs)] with and without global cAMP response element modulator (CREM) deletion (global-CREM KOs) to investigate the role of CREB in estrogen negative feedback on GnRH neurons. Evaluation of GnRH-CREB KO mice with and without global CREM deletion revealed normal puberty onset. Although estrus cycle length in adults was the same in controls and knock-out mice, cycles in mutant mice consisted of significantly longer periods of diestrus and less estrus. In GnRH-CREB KO mice, basal levels of luteinizing hormone (LH) and the postovariectomy increment in LH were normal, but the ability of E2 to rapidly suppress LH was significantly blunted. In contrast, basal and postovariectomy LH levels were abnormal in GnRH-CREB KO/global-CREM KO mice. Fecundity studies showed that GnRH-CREB KO with and without global CREM deletion were normal up to ∼9 months of age, at which time they became prematurely reproductively senescent. Morphological analysis of GnRH neurons revealed a significant reduction (p < 0.01) in GnRH somatic spine density of GnRH-CREB KO mice compared to control females. These observations implicate CREB within the GnRH neuron as an important target for E2's negative feedback actions. They also indicate that the rapid modulation of CREB by E2 is of physiological significance in the CNS.
Figures
Similar articles
-
Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility.Endocrinology. 2010 Sep;151(9):4428-36. doi: 10.1210/en.2010-0314. Epub 2010 Jun 23. Endocrinology. 2010. PMID: 20573723 Free PMC article.
-
Kisspeptin-gpr54 signaling at the GnRH neuron is necessary for negative feedback regulation of luteinizing hormone secretion in female mice.Neuroendocrinology. 2014;100(2-3):191-7. doi: 10.1159/000368608. Epub 2014 Dec 18. Neuroendocrinology. 2014. PMID: 25301053
-
Jak2 is necessary for neuroendocrine control of female reproduction.J Neurosci. 2011 Jan 5;31(1):184-92. doi: 10.1523/JNEUROSCI.2974-10.2011. J Neurosci. 2011. PMID: 21209203 Free PMC article.
-
Non-classical effects of estradiol on cAMP responsive element binding protein phosphorylation in gonadotropin-releasing hormone neurons: mechanisms and role.Front Neuroendocrinol. 2014 Jan;35(1):31-41. doi: 10.1016/j.yfrne.2013.08.002. Epub 2013 Aug 23. Front Neuroendocrinol. 2014. PMID: 23978477 Review.
-
Rapid actions of oestrogen on gonadotropin-releasing hormone neurons; from fantasy to physiology?J Physiol. 2009 Nov 1;587(Pt 21):5025-30. doi: 10.1113/jphysiol.2009.179838. Epub 2009 Aug 17. J Physiol. 2009. PMID: 19687121 Free PMC article. Review.
Cited by
-
Estradiol and Estrogen-like Alternative Therapies in Use: The Importance of the Selective and Non-Classical Actions.Biomedicines. 2022 Apr 6;10(4):861. doi: 10.3390/biomedicines10040861. Biomedicines. 2022. PMID: 35453610 Free PMC article. Review.
-
Deep sequencing of the transcriptome in the anterior pituitary of heifers before and after ovulation.J Vet Med Sci. 2017 Jun 10;79(6):1003-1012. doi: 10.1292/jvms.16-0531. Epub 2017 Apr 23. J Vet Med Sci. 2017. PMID: 28442638 Free PMC article.
-
Sex- and age-related changes in GABA signaling components in the human cortex.Biol Sex Differ. 2019 Jan 14;10(1):5. doi: 10.1186/s13293-018-0214-6. Biol Sex Differ. 2019. PMID: 30642393 Free PMC article.
-
Rodent Models of Non-classical Progesterone Action Regulating Ovulation.Front Endocrinol (Lausanne). 2017 Jul 24;8:165. doi: 10.3389/fendo.2017.00165. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28790975 Free PMC article. Review.
-
Perinatal exposure to the fungicide ketoconazole alters hypothalamic control of puberty in female rats.Front Endocrinol (Lausanne). 2023 Apr 3;14:1140886. doi: 10.3389/fendo.2023.1140886. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37077353 Free PMC article.
References
-
- Abrahám IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience. 2005;131:945–951. - PubMed
-
- Abrahám IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. - PubMed
-
- Barnea A, Gorski J. Estrogen-induced protein. Time course of synthesis. Biochemistry. 1970;9:1899–1904. - PubMed
-
- Blendy JA, Kaestner KH, Weinbauer GF, Nieschlag E, Schütz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
