Alzheimer's β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid
- PMID: 22895721
- PMCID: PMC3446780
- DOI: 10.1523/JNEUROSCI.0757-12.2012
Alzheimer's β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid
Abstract
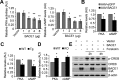
β-Amyloid protein (Aβ), the major component of neuritic plaques in Alzheimer's disease (AD), is derived from proteolytic cleavages of the amyloid precursor protein (APP) by β-site APP-cleaving enzyme 1 (BACE1) and the γ-secretase complex. BACE1 is the rate-limiting enzyme for Aβ production, and an increase in BACE1 level/activity contributes to the pathogenesis of sporadic AD. In addition to cleaving APP for Aβ generation, BACE1 plays multiple physiological roles including the regulation of synaptic functions. Here, we found that overexpression of BACE1 reduces cAMP response element binding protein (CREB) phosphorylation, protein kinase A (PKA) activity, and cAMP levels, whereas downregulation of BACE1 has the opposite effect. We showed that BACE1's effect is independent of its activity for Aβ production, which is corroborated by the observation that BACE1 transgenic mice have impaired learning/memory in the absence of neurotoxic human Aβ. Furthermore, we demonstrated that BACE1 interacts via its transmembrane domain with adenylate cyclase, resulting in reduction of cellular cAMP levels and thus PKA inactivation and reduced CREB phosphorylation. Our study suggests that in addition to its function as the β-secretase to produce Aβ, BACE1 may contribute to the memory and cognitive deficits typical of AD by regulating the cAMP/PKA/CREB pathway, which is important for memory functions.
Figures


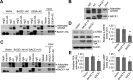
Similar articles
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
Regulation of Synaptic Amyloid-β Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease.J Neurosci. 2017 Mar 8;37(10):2639-2655. doi: 10.1523/JNEUROSCI.2851-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159908 Free PMC article.
-
Lamotrigine Reduces β-Site AβPP-Cleaving Enzyme 1 Protein Levels Through Induction of Autophagy.J Alzheimers Dis. 2015;46(4):863-76. doi: 10.3233/JAD-143162. J Alzheimers Dis. 2015. PMID: 25854934
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
-
Forsythoside A Mitigates Alzheimer's-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation.Int J Biol Sci. 2022 Feb 28;18(5):2075-2090. doi: 10.7150/ijbs.69714. eCollection 2022. Int J Biol Sci. 2022. PMID: 35342364 Free PMC article.
-
CREB signals as PBMC-based biomarkers of cognitive dysfunction: A novel perspective of the brain-immune axis.Brain Behav Immun. 2019 May;78:9-20. doi: 10.1016/j.bbi.2019.01.004. Epub 2019 Jan 12. Brain Behav Immun. 2019. PMID: 30641141 Free PMC article. Review.
-
Differentially Aquaporin 5 Expression in Submandibular Glands and Cerebral Cortex in Alzheimer's Disease.Biomedicines. 2022 Jul 8;10(7):1645. doi: 10.3390/biomedicines10071645. Biomedicines. 2022. PMID: 35884950 Free PMC article.
-
Neuroprotective Effect of 2-Aminoethoxydiphenyl Borate (2-APB) in Amyloid β-Induced Memory Dysfunction: A Mechanistic Study.Cell Mol Neurobiol. 2022 May;42(4):1211-1223. doi: 10.1007/s10571-020-01012-z. Epub 2020 Nov 21. Cell Mol Neurobiol. 2022. PMID: 33219878 Free PMC article.
-
Understanding PDE4's function in Alzheimer's disease; a target for novel therapeutic approaches.Biochem Soc Trans. 2019 Oct 31;47(5):1557-1565. doi: 10.1042/BST20190763. Biochem Soc Trans. 2019. PMID: 31642904 Free PMC article. Review.
References
-
- Bennett BD, Denis P, Haniu M, Teplow DB, Kahn S, Louis JC, Citron M, Vassar R. A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer's β-secretase. J Biol Chem. 2000;275:37712–37717. - PubMed
-
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. - PubMed
-
- Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–3912. - PMC - PubMed
-
- De Strooper B, Simons M, Multhaup G, Van Leuven F, Beyreuther K, Dotti CG. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J. 1995;14:4932–4938. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG035355/AG/NIA NIH HHS/United States
- R01 AG038710/AG/NIA NIH HHS/United States
- R01AG021173/AG/NIA NIH HHS/United States
- R01 AG021173/AG/NIA NIH HHS/United States
- R01 AG030197/AG/NIA NIH HHS/United States
- R01NS046673/NS/NINDS NIH HHS/United States
- P30 NS057096/NS/NINDS NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
- R01AG022074/AG/NIA NIH HHS/United States
- R01 NS046673/NS/NINDS NIH HHS/United States
- R01AG038710/AG/NIA NIH HHS/United States
- P01 NS074969/NS/NINDS NIH HHS/United States
- R01NS057096/NS/NINDS NIH HHS/United States
- R03AG034366/AG/NIA NIH HHS/United States
- R01 AG027924/AG/NIA NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- P01 AG030128/AG/NIA NIH HHS/United States
- R03 AG034366/AG/NIA NIH HHS/United States
- R21AG038968/AG/NIA NIH HHS/United States
- R01AG018840/AG/NIA NIH HHS/United States
- R21 AG038968/AG/NIA NIH HHS/United States
- R01AG030197/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
