Alternative splicing of the TRPC3 ion channel calmodulin/IP3 receptor-binding domain in the hindbrain enhances cation flux
- PMID: 22895723
- PMCID: PMC6621195
- DOI: 10.1523/JNEUROSCI.6446-11.2012
Alternative splicing of the TRPC3 ion channel calmodulin/IP3 receptor-binding domain in the hindbrain enhances cation flux
Abstract
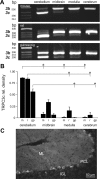
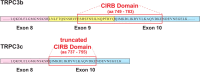
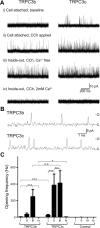
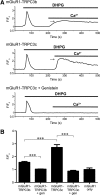
Canonical transient receptor potential (TRPC3) nonselective cation channels are effectors of G-protein-coupled receptors (GPCRs), activated via phospholipase C-diacylglycerol signaling. In cerebellar Purkinje cells, TRPC3 channels cause the metabotropic glutamate receptor (mGluR)-mediated slow EPSC (sEPSC). TRPC3 channels also provide negative feedback regulation of cytosolic Ca(2+), mediated by a C terminus "calmodulin and inositol trisphosphate receptor binding" (CIRB) domain. Here we report the alternative splicing of the TRPC3 mRNA transcript (designated TRPC3c), resulting in omission of exon 9 (approximately half of the CIRB domain) in mice, rats, and guinea pigs. TRPC3c expression is brain region specific, with prevalence in the cerebellum and brainstem. The TRPC3c channels expressed in HEK293 cells exhibit increased basal and GPCR-activated channel currents, and increased Ca(2+) fluorescence responses, compared with the previously characterized (TRPC3b) isoform when activated via either the endogenous M3 muscarinic acetylcholine receptor, or via coexpressed mGluR1. GPCR-induced TRPC3c channel opening rate (cell-attached patch) matched the maximum activation achieved with inside-out patches with zero cytosolic Ca(2+), whereas the GPCR-induced TRPC3b activation frequency was significantly less. Both TRPC3 channel isoforms were blocked with 2 mm Ca(2+), attributable to CIRB domain regulation. In addition, genistein blocked Purkinje cell (S)-2-amino-2-(3,5-dihydroxyphenyl) acetic acid (mGluR1)-activated TPRC3 current as for recombinant TRPC3c current. This novel TRPC3c ion channel therefore has enhanced efficacy as a neuronal GPCR-Ca(2+) signaling effector, and is associated with sensorimotor coordination, neuronal development, and brain injury.
Figures



Similar articles
-
Human Brain Region-Specific Alternative Splicing of TRPC3, the Type 3 Canonical Transient Receptor Potential Non-Selective Cation Channel.Cerebellum. 2019 Jun;18(3):536-543. doi: 10.1007/s12311-019-01026-4. Cerebellum. 2019. PMID: 30887370
-
A calmodulin/inositol 1,4,5-trisphosphate (IP3) receptor-binding region targets TRPC3 to the plasma membrane in a calmodulin/IP3 receptor-independent process.J Biol Chem. 2003 Jul 11;278(28):25758-65. doi: 10.1074/jbc.M303890200. Epub 2003 May 2. J Biol Chem. 2003. PMID: 12730194
-
Function and regulation of endothelin type A receptor-operated transient receptor potential canonical channels.J Pharmacol Sci. 2011;117(4):295-306. doi: 10.1254/jphs.11162fp. Epub 2011 Dec 1. J Pharmacol Sci. 2011. PMID: 22129540
-
Signalling mechanisms for TRPC3 channels.Novartis Found Symp. 2004;258:123-33; discussion 133-9, 155-9, 263-6. Novartis Found Symp. 2004. PMID: 15104179 Review.
-
TRPC3-dependent synaptic transmission in central mammalian neurons.J Mol Med (Berl). 2015 Sep;93(9):983-9. doi: 10.1007/s00109-015-1298-7. Epub 2015 Jun 5. J Mol Med (Berl). 2015. PMID: 26041382 Review.
Cited by
-
Diacylglycerol-mediated regulation of Aplysia bag cell neuron excitability requires protein kinase C.J Physiol. 2016 Oct 1;594(19):5573-92. doi: 10.1113/JP272152. Epub 2016 Jun 30. J Physiol. 2016. PMID: 27198498 Free PMC article.
-
Modulation and Regulation of Canonical Transient Receptor Potential 3 (TRPC3) Channels.Cells. 2023 Sep 5;12(18):2215. doi: 10.3390/cells12182215. Cells. 2023. PMID: 37759438 Free PMC article. Review.
-
Cerebellar modules operate at different frequencies.Elife. 2014 May 7;3:e02536. doi: 10.7554/eLife.02536. Elife. 2014. PMID: 24843004 Free PMC article.
-
The Moonwalker mouse: new insights into TRPC3 function, cerebellar development, and ataxia.Cerebellum. 2014 Oct;13(5):628-36. doi: 10.1007/s12311-014-0564-5. Cerebellum. 2014. PMID: 24797279 Free PMC article. Review.
-
Alternative splicing and nonsense-mediated mRNA decay enforce neural specific gene expression.Int J Dev Neurosci. 2016 Dec;55:102-108. doi: 10.1016/j.ijdevneu.2016.03.003. Epub 2016 Mar 8. Int J Dev Neurosci. 2016. PMID: 26968265 Free PMC article. Review.
References
-
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. - PubMed
-
- Chung YH, Sun Ahn H, Kim D, Hoon Shin D, Su Kim S, Yong Kim K, Bok Lee W, Ik Cha C. Immunohistochemical study on the distribution of TRPC channels in the rat hippocampus. Brain Res. 2006;1085:132–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
