In vivo reversible regulation of dendritic patterning by afferent input in bipolar auditory neurons
- PMID: 22895732
- PMCID: PMC3435435
- DOI: 10.1523/JNEUROSCI.1737-12.2012
In vivo reversible regulation of dendritic patterning by afferent input in bipolar auditory neurons
Abstract
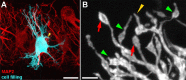
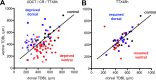
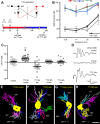
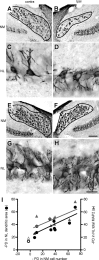
Afferent input regulates neuronal dendritic patterning locally and globally through distinct mechanisms. To begin to understand these mechanisms, we differentially manipulate afferent input in vivo and assess effects on dendritic patterning of individual neurons in chicken nucleus laminaris (NL). Dendrites of NL neurons segregate into dorsal and ventral domains, receiving excitatory input from the ipsilateral and contralateral ears, respectively, via nucleus magnocellularis (NM). Blocking action potentials from one ear, by either cochlea removal or temporary treatment with tetrodotoxin (TTX), leads to rapid and significant retraction of affected NL dendrites (dorsal ipsilaterally and ventral contralaterally) within 8 h compared with the other dendrites of the same neurons. The degree of retraction is comparable with that induced by direct deafferentation resulting from transection of NM axons. Importantly, when inner ear activity is allowed to recover from TTX treatments, retracted NL dendrites regrow to their normal length within 48 h. The retraction and growth involve elimination of terminal branches and addition of new branches, respectively. Examination of changes in NL dendrites at 96 h after unilateral cochlea removal, a manipulation that induces cell loss in NM and persistent blockage of afferent excitatory action potentials, reveals a significant correlation between cell death in the ipsilateral NM and the degree of dendritic retraction in NL. These results demonstrate that presynaptic action potentials rapidly and reversibly regulate dendritic patterning of postsynaptic neurons in a compartment specific manner, whereas long-term dendritic maintenance may be regulated in a way that is correlated with the presence of silent presynaptic appositions.
Figures


References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Benes FM, Parks TN, Rubel EW. Rapid dendritic atrophy following deafferentation: an EM morphometric analysis. Brain Res. 1977;122:1–13. - PubMed
-
- Born DE, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: neuron number and size following cochlea removal. J Comp Neurol. 1985;231:435–445. - PubMed
-
- Born DE, Durham D, Rubel EW. Afferent influences on brainstem auditory nuclei of the chick: nucleus magnocellularis neuronal activity following cochlea removal. Brain Res. 1991;557:37–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
