The effect of pre-incubation of Allium cepa L. roots in the ATH-rich extract on Pb uptake and localization
- PMID: 22895797
- PMCID: PMC3604584
- DOI: 10.1007/s00709-012-0445-z
The effect of pre-incubation of Allium cepa L. roots in the ATH-rich extract on Pb uptake and localization
Abstract
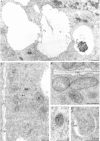
The positive influence of anthocyanin (ATH) on toxic metal-treated plant material is well documented; however, it is still not explained if it is caused by changes in element absorption and distribution. Therefore, detailed analysis of the effect of the ATH-rich extract from red cabbage leaves on Pb uptake and localization at morphological, anatomical and ultrastructural level was the goal of this study. Two-day-old adventitious roots of Allium cepa L. (cv. Polanowska) were treated for 2 h with the aqueous solution of Pb(NO3)2 at the concentration of 100 μM with or without preliminary incubation in the anthocyanin-rich extract from Brassica oleracea L. var. capitata rubra leaves (250 μM, 3 h). The red cabbage extract did not change the total Pb uptake but it enhanced the translocation of accumulated metal from roots to shoots. Within the pretreated roots, more Pb was deposited in their basal part and definitely smaller amount of the metal was bound in the apoplast of the outer layers of cortex cells. The ultrastructural analysis (transmission electron microscopy and X-ray microanalysis) revealed that the ATH-rich extract lowered the number of Pb deposits in intracellular spaces, cell wall and cytoplasm of root meristematic cells as well as in such organelles important to cell metabolism as mitochondria, plastids and nucleus. The Pb deposits were preferably localised in those vacuoles where ATH also occurred. This sequestration of Pb in vacuoles is probably responsible for reduction of metal cytotoxicity and consequently could lead to better plant growth.
Figures






References
-
- Alkorta I, Hernández Allica J, Becerri JM, Amezaga I, Albizu I, Garbisu C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsen. Rev Environ Sci Bio/Tech. 2004;3:71–90. doi: 10.1023/B:RESB.0000040059.70899.3d. - DOI
-
- Baranowska-Morek A, Wierzbicka M. Localization of lead in root tip of Dianthus carthusianorum. Acta Biol Cracov Bot. 2004;46:45–56.
-
- Brännvall ML, Bindler R, Renberg I, Emteryd O, Bartnicki J, Billström K. The Medieval metal industry was the cradle of modern large-scale atmospheric lead pollution in northern Europe. Environ Sci Technol. 1999;33:4391–4395. doi: 10.1021/es990279n. - DOI
-
- Buchauer MJ. Contamination of soil and vegetation near a zinc smelter by zinc, cadmium, copper and lead. Environ Sci Technol. 1973;7:131–135. doi: 10.1021/es60074a004. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

