Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial
- PMID: 22895835
- PMCID: PMC3583475
- DOI: 10.3892/or.2012.1956
Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial
Abstract
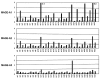
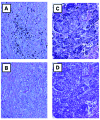
Metastatic and chemoresistant melanoma can be a good target of immunotherapy because it is an intractable cancer with a very poor prognosis. Previously, we tested a dendritic cell (DC)-based phase I vaccine, and confirmed that it was safe. In the present study, we performed a phase II trial of a DC vaccine for metastatic melanoma patients with mainly the HLA-A24 genotype, and investigated the efficacy of the vaccine. Twenty-four patients with metastatic melanoma were enrolled into a phase II study of DC-based immunotherapy. The group included 19 HLA-A24-positive (A*2402) patients and 3 HLA-A2-positive (A*0201) patients. The protocol for DC production was similar to that in the phase I trial. Briefly, a cocktail of 5 melanoma-associated synthetic peptides (gp100, tyrosinase, MAGE-A2, MAGE-A3 and MART-1 or MAGE‑A1) restricted to HLA-A2 or A24 and KLH were used for DC pulsing. Finally, DCs were injected subcutaneously (s.c.) into the inguinal region in the dose range of 1-5x107 per shot. The DC ratio (lin-HLA-DR+) of the vaccine was 38.1±13.3% and the frequency of CD83+ DCs was 25.7±20.8%. Other parameters regarding DC processing were not different from phase I. Immune response-related parameters including the ELISPOT assay, DTH reaction to peptide or KLH, DC injection numbers were shown to be related to a good prognosis. The ELISPOT reaction was positive in 75% of the patients vaccinated. The increase of anti-melanoma antigen antibody titer before vaccination was also shown to be a prognosis factor, but that post-vaccination was not. Based on immunohistochemical analysis, CD8 and IL-17 were not involved in the prognosis. Adverse effects of more than grade III were not seen. Overall survival analysis revealed a significant survival prolongation effect in DC-given melanoma patients. These results suggest that peptide cocktail-treated DC vaccines may be a safe and effective therapy against metastatic melanoma in terms of prolongation of overall survival time.
Figures

References
-
- Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB. Bone marrow-derived dendritic cells pulsed with synthetic tumor peptides elicit protective and therapeutic antitumor immunity. Nat Med. 1995;1:1297–1302. - PubMed
-
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide-or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. - PubMed
-
- Ranieri E, Kierstead LS, Zarour H, Kirkwood JM, Lotze MT, Whiteside T, Storkus WJ. Dendritic cell/peptide cancer vaccine: clinical responsiveness and epitope spreading. Immunol Invest. 2000;29:121–125. - PubMed
-
- Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, et al. Vaccination with Mage-3a1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

