Hormone therapy in postmenopausal women and risk of endometrial hyperplasia
- PMID: 22895916
- PMCID: PMC7039145
- DOI: 10.1002/14651858.CD000402.pub4
Hormone therapy in postmenopausal women and risk of endometrial hyperplasia
Update in
-
Hormone therapy in postmenopausal women and risk of endometrial hyperplasia or endometrial cancer.Cochrane Database Syst Rev. 2025 Oct 23;10(10):CD000402. doi: 10.1002/14651858.CD000402.pub5. Cochrane Database Syst Rev. 2025. PMID: 41128095
Abstract
Background: Reduced circulating estrogen levels around the time of the menopause can induce unacceptable symptoms that affect the health and well-being of women. Hormone therapy (both unopposed estrogen and estrogen/progestogen combinations) is an effective treatment for these symptoms, but is associated with risk of harms. Guidelines recommend that hormone therapy be given at the lowest effective dose and treatment should be reviewed regularly. The aim of this review is to identify the minimum dose(s) of progestogen required to be added to estrogen so that the rate of endometrial hyperplasia is not increased compared to placebo.
Objectives: The objective of this review is to assess which hormone therapy regimens provide effective protection against the development of endometrial hyperplasia or carcinoma.
Search methods: We searched the Cochrane Menstrual Disorders and Subfertility Group trials register (searched January 2012), The Cochrane Library (Issue 1, 2012), MEDLINE (1966 to January 2012), EMBASE (1980 to January 2012), Current Contents (1993 to May 2008), Biological Abstracts (1969 to 2008), Social Sciences Index (1980 to May 2008), PsycINFO (1972 to January 2012) and CINAHL (1982 to May 2008). Attempts were made to identify trials from citation lists of reviews and studies retrieved, and drug companies were contacted for unpublished data.
Selection criteria: Randomised comparisons of unopposed estrogen therapy, combined continuous estrogen-progestogen therapy, sequential estrogen-progestogen therapy with each other or placebo, administered over a minimum period of 12 months. Incidence of endometrial hyperplasia/carcinoma assessed by a biopsy at the end of treatment was a required outcome. Data on adherence to therapy, rates of additional interventions, and withdrawals owing to adverse events were also extracted.
Data collection and analysis: In this update, 46 studies were included. Odds ratios (ORs) were calculated for dichotomous outcomes. The small numbers of studies in each comparison and the clinical heterogeneity precluded meta-analysis for many outcomes.
Main results: Unopposed estrogen is associated with increased risk of endometrial hyperplasia at all doses, and durations of therapy between one and three years. For women with a uterus the risk of endometrial hyperplasia with hormone therapy comprising low-dose estrogen continuously combined with a minimum of 1 mg norethisterone acetate (NETA) or 1.5 mg medroxyprogesterone acetate (MPA) is not significantly different from placebo at two years (1 mg NETA: OR 0.04; 95% confidence interval (CI) 0 to 2.8; 1.5 mg MPA: no hyperplasia events).
Authors' conclusions: Hormone therapy for postmenopausal women with an intact uterus should comprise both estrogen and progestogen to reduce the risk of endometrial hyperplasia.
Conflict of interest statement
There was no conflict of interest.
Figures

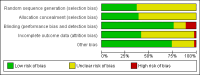



























































Update of
-
Hormone therapy in postmenopausal women and risk of endometrial hyperplasia.Cochrane Database Syst Rev. 2009 Apr 15;(2):CD000402. doi: 10.1002/14651858.CD000402.pub3. Cochrane Database Syst Rev. 2009. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD000402. doi: 10.1002/14651858.CD000402.pub4. PMID: 19370558 Updated.
References
References to studies included in this review
AinMelk 1996 {published data only}
-
- AinMelk Y. Comparison of two continuous combined estrogen progestogen regimens in postmenopausal women: a randomised trial. Fertility & Sterility 1996;66(6):962‐8. - PubMed
Al‐Azzawi 2001 {published data only}
-
- Al‐Azzawi F, Wahab M, Thompson J, Pornel B, Hirvonen E, Ylikorkala O, et al. Acceptability and patterns of endometrial bleeding in estradiol‐based HRT regimens: a comparative study of cyclical sequential combinations of trimegestone or norethisterone acetate. Climacteric 2001;4(4):343‐54. - PubMed
Archer 2005 {published data only}
-
- Archer DF, Thorneycroft IH, Foegh M, Hanes V, Glant MD, Bitterman P, et al. Long‐term safety of drospirinone‐estradiol for hormone therapy: a randomised, double‐blind, multicenter trial. Menopause 2005;12(6):716‐27. - PubMed
Bouchard 2005 {published data only}
-
- Bouchard P, Addo S, Spielmann D, The Trimegestone 301 Study Group. A comparison of the effects of continuous combined regimens of 1mg 17B‐estradiol and trimegestone with continuous combined estradiol and norethisterone acetate upon the bleeding profile and safety in postmenopausal women for up to 2 years. Climacteric. 2002; Vol. 5, issue Suppl 1:145.
-
- Bouchard P, Cicco‐Nardone F, Spielmann D, Garcea N, The Trimegestone 301 Study Group. Bleeding profile and endometrial safety of continuous combined regimens 1 mg 17beta‐estradiol/trimegestone versus 1 or 2 mg 17beta‐estradiol/norethisterone acetate in postmenopausal women. Gynecological Endocrinology 2005;21(3):142‐8. - PubMed
-
- Gambacciani M, Spielmann D, Genazzani AR. Efficacy on climacteric symptoms of a continuous combined regimen of 1 mg 17beta‐estradiol and trimegestone versus two regimens combining 1 or 2 mg 17beta‐estradiol and norethisterone acetate. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology 2005;21(2):65‐73. - PubMed
Bruhat 2001 {published data only}
-
- Bruhat M, Rudolf K, Vaheri R, Kainulainen P, Timonen U, Viitanen A. Effective bleeding control and symptom relief by lower dose regimens of continuous combined hormone replacement therapy: a randomized comparative dose‐ranging study. Maturitas 2001;40:259‐71. - PubMed
Byrjalsen 1992 {published data only}
-
- Byrjalsen I, Thormann L, Meinecke B, Riis BJ, Christiansen C. Sequential estrogen and progestogen therapy: assessment of progestational effects on the postmenopausal endometrium. Obstetrics & Gynecology 1992;79(4):523‐8. - PubMed
Byrjalsen 1999 {published data only}
-
- Byrjalsen I, Bjarnason NH, Christiansen C. Progestational effects of combinations of gestodene on the postmenopausal endometrium during hormone replacement therapy. Acta Obsterica et Gynecologica Scandinavica 1997;76(Suppl):19. - PubMed
-
- Byrjalsen I, Bjarnason NH, Christiansen C. Progestational effects of combinations of gestodene on the postmenopausal endometrium during hormone replacement therapy. American Journal of Obstetrics & Gynecology 1999;180(3 I):539‐49. - PubMed
Byrjalsen 2000 {published data only}
-
- Byrjalsen I, Alexandersen P, Christiansen C. Piperazine oestrone sulphate and interrupted norethisterone: effects on the postmenopausal endometrium. BJOG: an International Journal of Obstetrics & Gynaecology 2000;107(3):347‐55. - PubMed
Chang 2003 {published data only}
-
- Chang TC, Chen M, Lien YR, Chen RJ, Chow SN. Comparison of the difference in histopathology and cell cycle kinetics among the postmenopausal endometrium treated with different progestins in sequential‐combined hormone replacement therapy. Menopause 2003;10(2):172‐8. - PubMed
CHART 1996 {published data only}
-
- Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium and lipids of continuous hormones as replacement therapy (CHART study). A randomised controlled trial. JAMA 1996;276(7):1397‐403. - PubMed
Corson 1999 {published data only}
-
- Corson S, Richart R, Caubel P, Lim P. Effect of a unique constant estrogen, pulsed progestin hormone replacement therapy containing 17B estradiol and norgestimate on endometrial histology. International Journal of Fertility 1999;44:279‐85. - PubMed
-
- Sulak P, Caubel P, Lane R. Efficacy and safety of constant estrogen, pulsed progestin regimen in hormone replacement therapy. International Journal of Fertility 1999;44:286‐96. - PubMed
Ettinger 1992 {published data only}
-
- Ettinger B, Genant HK, Steiger P, Madvig P. Low‐dosage micronized 17B‐estradiol prevents bone loss in postmenopausal women. American Journal of Obstetrics and Gynecology 1992;166:479‐88. - PubMed
Ferenczy 2002 {published data only}
-
- Ferenczy A, Gelfand MM, Weijer PHM, Rioux JE. Endometrial safety and bleeding patterns during a 2 year study of 1 or 2 mg of 17B‐estradiol combined with sequential 5‐20mg dydrogesterone. Climacteric 2002;5:26‐35. - PubMed
Gelfand 1989 {published data only}
-
- Gelfand M, Ferenczy A. A prospective 1‐year study of estrogen and progestin in postmenopausal women: effects on the endometrium. Obstetrics & Gynecology 1989;74:398‐402. - PubMed
Graser 2000 {published data only}
-
- Graser T, Koytchev R, Muller A, Oettel M. Comparison of the efficacy and endometrial safety of two estradiol valerate/dienogest combinations and Kliogest for continuous combined hormone replacement therapy in postmenopausal women. Climacteric 2000;3(2):109‐18. - PubMed
Greenwald 2005 {published data only}
-
- Greenwald MW, Gluck OS, Lang E, Rakov V. Oral hormone therapy with 17s‐estradiol and 17s‐estradiol in combination with norethindrone acetate in the prevention of bone loss in early postmenopausal women: dose‐dependent effects. Menopause 2005;12(6):741‐8. - PubMed
Harris 1991 {published data only}
-
- Harris ST, Genant HK, Baylink DJ, Gallagher C, Karp SK, McConnell MA, et al. The effects of estrone (OGEN) on spinal bone density of postmenopausal women. Archives of Internal Medicine 1991;151:1980‐4. - PubMed
Heikkinen 1997 {published data only}
-
- Heikkinen J, Kyllonen E, Kurttila‐Matero E, Wilen‐Rosenqvist G, Lankinen KS, Rita H, et al. HRT and exercise: effects on bone density, muscle strength and lipid metabolism. A placebo controlled 2‐year prospective trial on two estrogen‐progestin regimens in healthy postmenopausal women. Maturitas 1997;26:139‐49. - PubMed
-
- Heikkinen JE, Finney JS, Lankinen KS, Wilen‐Rosenqvist GH. Comparison of bleeding patterns and endometrial histology between a three monthly and monthly cycle HRT (abstract). Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167):P76.33.
Heikkinen 2000 {published data only}
-
- Heikkinen J, Vaheri R, Kainulainen P, Timonen U. Long‐term continuous combined hormone replacement therapy in the prevention of postmenopausal bone loss: a comparison of high‐ and low‐dose estrogen‐progestin regimens. Osteoporosis International 2000;11(11):929‐37. - PubMed
-
- Heikkinen J, Vaheri R, Maenpaa J, Timonen U. Long‐term endometrial safety of continuous combined HRT. Results from a 7‐year randomised study. International Journal of Obstetrics & Gynecology 2003;83(Suppl 3):98‐9.
-
- Heikkinen J, Vaheri R, Timonen U. A 10‐year follow‐up of postmenopausal women on long‐term continuous combined hormone replacement therapy: update of safety and quality‐of‐life findings [erratum appears in Journal of the British Menopause Society 2006;12(4):174]. Journal of the British Menopause Society 2006; Vol. 12, issue 3:115‐25. - PubMed
-
- Heikkinen J, Vaheri R, Timonen U. Long‐term safety and tolerability of continuous‐combined hormone therapy in postmenopausal women: results from a seven‐year randomised comparison of low and standard doses. Journal of the British Menopause Society 2004;10(3):95‐102. - PubMed
-
- Heikkinen JE, Vaheri RT, Ahomaki SM, Kainulainen PM, Viitanen AT, Timonen UM. Optimizing continuous‐combined hormone replacement therapy for postmenopausal women: a comparison of six different treatment regimens. American Journal of Obstetrics & Gynecology 2000;182(3):560‐7. - PubMed
HOPE 2001 {published data only}
-
- Archer D, Lobo R, Utian W, Pickar J. Improved amenorrhoea, favourable vasomotor and lipid effects, and endometrial safety with lower doses of conjugated estrogens (CE) and medroxyprogesterone acetate (MPA). Gynecological Endocrinology 2000;14(Suppl 2):239.
-
- Archer DF, Dorin M, Lewis V, Schneider DL, Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on endometrial bleeding. Fertility & Sterility 2001;75(6):1080‐7. - PubMed
-
- Pickar J, Tien Yeh I, Wheeler J, Cunnane M, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertility & Sterility 2001;76:25‐31. - PubMed
-
- Pickar J, Yeh I, Wheeler J, Cunnane M, Speroff L. Endometrial safety with lower doses of conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA). Fertility & Sterility. 2001; Vol. 76, issue 3S:52. - PubMed
-
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two‐year substudy results. Fertility & Sterility 2003;80(5):1234‐40. - PubMed
Koninckx 2005 {published data only}
-
- Koninckx P, Spielmann D, The Trimegestone 302 Study Group. A comparative 2‐year study of two sequential regimens of 1mg estradiol and trimegestone with 1mg estradiol and norethisterone upon profiles of endometrial bleeding and safety in postmenopausal women. Climacteric 2002;5(Suppl 1):144. - PubMed
-
- Koninckx PR, Spielmann D. A comparative 2‐year study of the effects of sequential regimens of 1 mg 17beta‐estradiol and trimegestone with a regimen containing estradiol valerate and norethisterone on the bleeding profile and endometrial safety in postmenopausal women. Gynecological Endocrinology 2005;21(2):82‐9. - PubMed
-
- Pornel B, Spielman D, The Trimgestone 302 Study Group. A study of the control of climacteric symptoms in postmenopausal women following sequential regimens of 1mg 17 B‐estradiol and trimegestone compared with a regimen containing 1mg estradiol valerate and norethisterone over a 2 year period. Gynaecological Endocrinology 2005;21(2):74‐81. - PubMed
Kurman 2000 {published data only}
-
- Kurman J, Carlos Felix J, Archer D, Nanavati N, Arce JC, Moyer D. Norethindrone acetate and estradiol‐induced endometrial hyperplasia. Obstetrics & Gynecology 2000;96(3):373‐9. - PubMed
Luciano 1993 {published data only}
-
- Luciano AA, Souza MJ, Roy MP, Schoenfeld MJ, Nulsen JC, Halvorson CV. Evaluation of low‐dose estrogen and progestin therapy in postmenopausal women. Journal of Reproductive Medicine 1993;38(3):207‐14. - PubMed
Mattsson 2004 {published data only}
-
- Mattsson LA, Skouby SO, Heikkinen J, Vaheri R, Maenpaa J, Timonen U. A low‐dose start in hormone replacement therapy provides a beneficial bleeding profile and few side‐effects: randomized comparison with a conventional‐dose regimen. Climacteric 2004;7(1):59‐69. - PubMed
-
- Vaheri R, Matsson LA, Skouby S, Heikkinen J, Maenpaa J. Bleeding control, endometrial safety and tolerability of various continuous combined HRT regimens (abstract). Climacteric 2002;5(Suppl 1):146.
Meuwissen 2001 {published data only}
-
- Meuwissen JH, Beijers‐De Bie L, Vihtamaki T, Tuimala R, Siseles N, Magaril C, et al. A 1‐year comparison of the efficacy and clinical tolerance in postmenopausal women of two hormone replacement therapies containing estradiol in combination with either norgestrel or trimegestone. Gynecological Endocrinology 2001;15(5):349‐58. - PubMed
MSG 1994 {published data only}
-
- Archer D, Pickar J. Effects of progestin dose and time since menopause on endometrial bleeding with continuous combine hormone replacement therapy. International Journal of Obstetrics and Gynecology 2000;70(Suppl 3):120. - PubMed
-
- Archer DF, Pickar JH. Hormone replacement therapy: effect of progestin dose and time since menopause on endometrial bleeding. Obstetrics & Gynecology 2000;96(6):899‐905. - PubMed
-
- Archer DF, Picker JH, Bottiglioni F for the Menopause Study Group. Bleeding patterns in postmenopausal women taking continuous combined or sequential regimens of conjugated estrogens with medroxyprogesterone acetate. Obstetrics and Gynecology 1994;83(5):686‐92. - PubMed
-
- Pickar JH, Archer DF. Is bleeding a predictor of endometrial hyperplasia in postmenopausal women receiving hormone replacement therapy. American Journal of Obstetrics and Gynecology 1997;177:1178‐83. - PubMed
-
- Woodruff JD, Pickar JH for the Menopause Study Group. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. American Journal of Obstetrics and Gynecology 1994;170(5):1213‐23. - PubMed
Nand 1995 {published data only}
-
- Nand SL, Webster MA, Wren BG. Continuous combined piperazine oestrone sulphate and medroxyprogesterone acetate hormone replacement therapy ‐ a study of bleeding pattern, endometrial response, serum lipid and bone density changes. Australian & New Zealand Journal of Obstetrics & Gynaecology 1995;35(1):92‐6. - PubMed
Notelovitz 1997 {published data only}
-
- Genant HK, Lucas J, Weiss S, Akin M, Emkey R, McNaney‐Flint H, et al. Low‐dose esterified estrogen therapy. Effects on bone, plasma estradiol concentrations, endometrium and lipid levels. Archives of Internal Medicine 1997;157:2609‐15. - PubMed
-
- Lucas J, Rebar R, Speroff L, Trabal J, Silfen S. A two‐year study with low dose, continuous, unopposed esterified estrogens: prevention of postmenopausal bone loss with minimal endometrial effects. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sydney, Australia, 1996:212.
-
- Notelovitz M, Varner RE, Rebar RW, Fleischmann R, McIlwain HH, Schwartz SL, et al. Minimal endometrial proliferation over a two‐year period in postmenopausal women taking 0.3mg of unopposed esterified estrogens. Menopause 1997;4(2):80‐8.
-
- Trabal JF, Lenihan JP, Melchione TE, Stoltz RR, Khairi S, Yang H‐M, et al. Low‐dose unopposed estrogens: preliminary findings on the frequency and duration of vaginal bleeding in postmenopausal women receiving esterified estrogens over a two‐year period. Menopause 1997;4(3):130‐8.
Obel 1993 {published data only}
-
- Obel EB, Munk‐Jensen N, Svenstrup B, Bennett P, Micic S, Henrik‐Nielsen R, et al. A two‐year double‐blind controlled study of the clinical effect of combined and sequential postmenopausal replacement therapy and steroid metabolism during treatment. Maturitas 1993;16:13‐21. - PubMed
OGEN‐Provera 1998 {published data only}
-
- Nand S, Webster M, Baber R, O'Connor V. Bleeding pattern and endometrial changes during continuous combined hormone replacement therapy. Obstetrics & Gynecology 1998;91:678‐84. - PubMed
-
- Webster M. Combination continuous therapy ‐ what is the optimal MPA dose? Effect on bleeding profiles and endometrial histology. Proceedings of the 8th International Congress on the Menopause, 1996 Nov 3‐7. Sydney, Australia, 1996:27.
Okon 2001 {published data only}
-
- Okon MA, Lee S, Laird SM, Li TC. A prospective randomised controlled study comparing two doses of gestodene in cyclic combined HRT preparations on endometrial physiology. Human Reproduction 2001;16(6):1244‐50. - PubMed
OPAL 2006 {published data only}
-
- Bots ML, Evans GW, Riley W, McBride KH, Paskett ED, Helmond FA, et al. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima‐media thickness: the Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. European Heart Journal 2006;27(6):746‐55. - PubMed
-
- Bots ML, Evans GW, Riley W, Meijer R, McBride KH, Paskett ED, et al. The Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study: design and baseline characteristics. Controlled Clinical Trials 2003;24(6):752‐75. - PubMed
-
- Langer RD, Landgren BM, Rymer J, Helmond FA. Effects of tibolone and continuous combined conjugated equine estrogen/medroxyprogesterone acetate on the endometrium and vaginal bleeding: results of the OPAL study. American Journal of Obstetrics and Gynecology 2006;195(5):1320‐7. - PubMed
PEPI 1995 {published and unpublished data}
-
- Barrett‐Connor E, Espeland MA, Greendale GA, Trabal J, Johnson S, Legault C, et al. Postmenopausal hormone use following a 3‐year randomised clinical trial. Journal of Womens Health & Gender‐Based Medicine 2000;9(6):633‐43. - PubMed
-
- Bush TL, Espeland MA, Mebane‐Sims I. The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. Overview. Controlled Clinical Trials 1995;16:1S‐2S. - PubMed
-
- Espeland MA, Bush TL, Mebane‐Sims I, Stefanick ML, Johnson S, Sherwin R, et al. Rationale, design, and conduct of the PEPI Trial. Controlled Clinical Trials 1995;16:3S‐19S. - PubMed
-
- Johnson S, Mebane‐Sims I, Hogan PE, Stoy DB. Recruitment of postmenopausal women in the PEPI Trial. Controlled Clinical Trials 1995;16:20S‐35S. - PubMed
-
- Lindenfield E, Langer R. Bleeding patterns of the hormone replacement therapies in postmenopausal estrogen and progestin interventions trial. Obstetrics and Gynecology 2002;100:853‐63. - PubMed
Portman 2003 {published data only}
-
- Portman DJ, Shumel BS, Symons JP. Hormone replacement therapy (HRT) with 1 mg norethindrone acetate (NA) / 5 mcg ethinyl estradiol (EE) (FemHRT) provides greater protection against breakthrough bleeding versus 0.625 mg combined equine estrogens (CEE) / 2.5 mg medroxyprogesterone (MPA) (Prempro). The 3rd World Congress on Controversies in Obstetrics, Gynecology & Infertility. 2002:82.
-
- Portman DJ, Symons JP, Wilborn W, Kempfert NJ. A randomised, double‐blind, placebo‐controlled, multicenter study that assessed the endometrial effects of norethindrone acetate plus ethinyl estradiol versus ethinyl estradiol alone. American Journal of Obstetrics & Gynecology 2003;188(2):334‐42. - PubMed
Prestwood 2003 {published data only}
-
- Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultra‐low‐dose micronized 17beta‐estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA 2003;290(8):1042‐8. - PubMed
Rees 2004 {published data only}
-
- Rees MCP, Kuhl H, Engelstein M, Mattila L, Maenpaa J, Mustonen M. Endometrial safety and tolerability of triphasic sequential hormone replacement estradiol valerate/medroxyprogesterone acetate therapy regimen. Climacteric 2004;7(1):23‐32. - PubMed
Rozenberg 2001 {published data only}
-
- Rozenberg S, Caubel P, Lim PC. Constant estrogen, intermittent progestogen vs continuous combined hormone replacement: tolerability and effect on vasomotor symptoms. International Journal of Gynecology and Obstetrics 2001;72:235‐43. - PubMed
-
- Rozenberg S, Lim P. One‐year efficacy and safety of Prefect, a pulsed progestogen, constant estrogen hormone replacement therapy regimen. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1999;86(Suppl):S18‐9.
-
- Ylikorkala O, Lim P, Caubel P. Effects on serum lipid profiles of continuous 17beta‐estradiol, intermittent norgestimate regimens versus continuous combined 17beta‐estradiol/norethisterone acetate hormone replacement therapy. Clinical Therapeutics 2000; Vol. 22, issue 5:622‐36. - PubMed
-
- Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long‐term comparison between estradiol 1 mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstetricia et Gynecologica Scandinavica 2002;81:654‐9. - PubMed
Scandinavia 1996 {published and unpublished data}
-
- Bjarnason K, Cerin A, Lingren R, Weber T. Adverse endometrial effects during long cycle hormone replacement therapy. Maturitas 1999;32:161‐70. - PubMed
-
- Cerin A, Heldaas K, Moeller B for the Scandinavian Long Cycle Study Group. Adverse endometrial effects of long‐cycle estrogen and progestogen replacement therapy (letter). New England of Journal of Medicine 1996;334(10):668‐9. - PubMed
-
- Cerin A, Hjarnason K, Heldaas K, Thomsen HK, Boeller B. Adverse endometrial effects during long cycle hormone replacement therapy. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sydney, Australia, 1996:48.
Sporrong 1988 {published data only}
-
- Sporrong T, Hellgren M, Samsioe G, Mattsson LA. Comparison of four continuously administered progestogen plus oestradiol combinations for climacteric complaints. British Journal of Obstetrics and Gynaecology 1988;95(10):1042‐8. - PubMed
-
- Sporrong T, Samsioe G, Larsen S, Mattsson LA. A novel statistical approach to analysis of bleeding patterns during continuous hormone replacement therapy. Maturitas 1989;11(3):209‐15. - PubMed
Stadberg 1996 {published data only}
-
- Stadberg E, Mattsson LA, Uvebrant M. 17 beta‐estradiol and norethisterone acetate in low doses as continuous combined hormone replacement therapy. Maturitas 1996;23(1):31‐9. - PubMed
van de Weijer 1999 {published data only}
-
- Weijer PH, Scholten PC, Mooren MJ, Barentsen R, Kenemans P. Bleeding patterns and endometrial histology during administration of low‐dose estradiol sequentially combined with dydrogesterone. Climacteric 1999;2(2):101‐9. - PubMed
Warming 2004 {published data only}
-
- Warming L, Ravn P, Nielsen T, Christiansen C. Safety and efficacy of drospirinone used in a continuous combination with 17beta‐estradiol for prevention of postmenopausal osteoporosis. Climacteric 2004;7(1):103‐11. - PubMed
WHI 2002 {published data only}
-
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SAA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial.[see comment]. JAMA 2003; Vol. 290, issue 13:1739‐48. - PubMed
-
- Barnabei VM, Cochrane BB, Aragaki AK, Nygaard I, Williams RS, McGovern PG, et al. Menopausal symptoms and treatment‐related effects of estrogen and progestin in the Women's Health Initiative. Obstetrics & Gynecology 2005;105(5 Pt 1):1063‐73. - PubMed
-
- Writing group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA 2002;288:321‐33. - PubMed
Williams 1994 {published data only}
-
- Williams DB, Voigt BJ, Fu YS, Schoenfeld MJ, Judd HL. Assessment of less than monthly progestin therapy in postmenopausal women given estrogen replacement. Obstetrics & Gynecology 1994;84(5):787‐93. - PubMed
Wu 2002 {published data only}
-
- Wu Y, Liu J, Xing S, Xu R, Zhang Z, Wang Y. Comparative study on two different dosages of conjugated equine estrogen continuously combined with medroxyprogesterone in prevention of postmenopausal osteoporosis. Chinese Journal of Obstetrics & Gynecology 2002;37(5):267‐70. - PubMed
-
- Xing S, Wu Y, Liu J, Xu R, Zhang Z, Wang Y. A comparison of two different dosages of conjugated equine estrogen in continuous combined hormone replacement therapy with progestin. Chinese Medical Journal 2003;116(4):584‐7. - PubMed
Yildirim 2006 {published data only}
-
- Yildirim G, Tugrul S, Uslu H, Pekin O, Eren S. Effects of two different regimens of continuous hormone replacement therapy on endometrial histopathology and postmenopausal uterine bleeding. Archives of Gynecology & Obstetrics 2006;273(5):268‐73. - PubMed
References to studies excluded from this review
Aoki 1990 {published data only}
-
- Aoki T, Asai T. Assessment of oestrogen replacement therapy with conjugated oestrogens by histological evaluation of the endometrium in 205 women. Proceedings of the 6th International Congress on the Menopause, Bangkok, Thailand, 1990, Oct 29‐Nov 3. Bangkok, Thailand, 1990:151.
Archer 1999 {published data only}
-
- Archer D, Dorin M, Heine W, Nanavati N, Arce J. Uterine bleeding in postmenopausal women on continuous therapy with estradiol and norethindrone acetate. Obstetrics and Gynecology 1999;94:323‐9. - PubMed
Archer 2001 {published data only}
-
- Archer D, Dorin M, Lewis V, Schneider D, Pickar J. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on endometrial bleeding. Fertility & Sterility 2001;75(6):1080‐7. - PubMed
Arrenbrecht 2004 {published data only}
-
- Arrenbrecht S, Caubel P, Garnero P, Felsenberg D, Vogel N, Coenders A, et al. The effect of continuous oestradiol with intermittent norgestimate on bone mineral density and bone turnover in post‐menopausal women. Maturitas 2004;48(3):197‐207. - PubMed
Bergeron 2010 {published data only}
-
- Bergeron C, Nogales FF, Rechberger T, Tatarchjuk T, Zipfel L. Ultra low dose continuous combined hormone replacement therapy with 0.5mg 17beta ‐oestradiol and 2.5mg dydrogesterone: protection of the endometrium and amenorrhoea rate. Maturitas 2010; Vol. 66, issue 2:201‐5. - PubMed
Blumel 1994 {published data only}
-
- Blumel JE, Roncagliolo ME, Gramegna G, Vasquez R, Estartus A, Tacla X, et al. Double‐blind study of the effect of continuous therapy with estradiol valerate and medroxyprogesterone acetate on menopausal symptoms, lipid profile and endometrial thickness [Estudio doble ciego del efecto sobre la sintomatologia menopausica, el perfil lipidico y el grosor endometrial de una terapia continua de valerato de estradiol mas acetato de medroxiprogesterona]. Revista Chilena Obstetricia y Ginecologia 1994;59(5):354‐60. - PubMed
Byrjalsen 1992a {published data only}
-
- Byrjalsen I, Thormann L, Riis BJ, Christiansen. Secretory endometrial protein PP14 in serum from post‐menopausal women receiving continuous combined oestradiol‐cyproterone acetate: correlation with serum hormone concentrations and bleeding patterns. Maturitas 1992;15:39‐46. - PubMed
Byrjalsen 1992b {published data only}
-
- Byrjalsen I, Thormann L, Meinecke B, Riis BJ, Christiansen C. Serum placental protein 14 (PP14) reflects endometrial status during hormone replacement therapy. Human Reproduction 1992; Vol. 7, issue 8:1042‐7. - PubMed
Campbell 1977 {published data only}
-
- Campbell S, Whitehead M. Oestrogen therapy and the menopausal syndrome. Clinics in Obstetrics and Gynaecology 1977;4(1):31‐47. - PubMed
Campodonico 1996 {published data only}
-
- Campodonico I, Valdivia I. Bleeding pattern in two combined continuous hormone replacement therapy (HRT) regimes in climacteric women. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sydney, Australia, 1996:191.
Chen 1999 {published data only}
-
- Chen FP, Teng LF. Effects of cyclic continuous and sequential post‐menopausal hormone replacement therapy on uterine bleeding and climacteric symptoms. Human Reproduction 1999;14:246.
Christensen 1982 {published data only}
-
- Christensen MS, Hagen C, Christiansen C, Transbol I. Dose‐response evaluation of cyclic estrogen/gestagen in postmenopausal women: placebo‐controlled trial of its gynaecologic and metabolic actions. American Journal of Obstetrics & Gynecology 1982;144(8):873‐9. - PubMed
Endrikat 2007 {published data only}
-
- Endrikat J, Graeser T, Mellinger U, Ertan K, Holz C, Endrikat J, et al. A multicenter, prospective, randomized, double‐blind, placebo‐controlled study to investigate the efficacy of a continuous‐combined hormone therapy preparation containing 1mg estradiol valerate/2mg dienogest on hot flushes in postmenopausal women. Maturitas 2007; Vol. 58, issue 2:201‐7. - PubMed
Granberg 2002 {published data only}
-
- Granberg S, Eurenius K, Lindgren R, Wilhelmsson L. The effects of oral estriol on the endometrium in postmenopausal women. Maturitas 2002;42:149‐56. - PubMed
Gulhan 2004 {published data only}
-
- Gulhan S, Dilek S. The effect of different postmenopausal hormone replacement treatment protocols on endometrial thickness. Gulhane Medical Journal 2004;46(3):205‐8.
Hagen 1982 {published data only}
-
- Hagen C, Christensen MS, Christiansen C, Stocklund K‐E, Transbol I. Effects of two years' estrogen‐gestagen replacement on climacteric symptoms and gonadotropins in the early postmenopausal period. Acta Obstetrica et Gynecologica Scandinavica 1982;61:237‐41. - PubMed
Heytmanek 1990 {published data only}
-
- Heytmanek G. Continuous hormone replacement therapy with oestradiol valerate alone and in combination with cyproterone acetate: clinical and endometrial findings. Proceedings of the 6th International Congress on the Menopause, Bangkok, Thailand, 1990, Oct 29‐Nov 3. Bangkok, Thailand, 1990:86.
Istre 1996 {published data only}
-
- Istre O, Holm Nielsen P, Bourne T, Forman A. Hormone replacement therapy after transcervical resection of the endometrium. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:100. - PubMed
-
- Istre O, Holm‐Nielsen P, Bourne T, Forman A. Hormone replacement therapy after transcervical resection of the endometrium. Obstetrics & Gynecology 1996;88(5):767‐70. - PubMed
Jaisamrarn 2002 {published data only}
-
- Jaisamrarn S, Sitravarin N, Taechakrichana K, Panyakamlert K, Limpapayom K. Bleeding patterns in menopausal women taking HRT. Climacteric 2002;F‐13‐03:75.
Jirapinyo 2003 {published data only}
-
- Jirapinyo M, Theppisai U, Manonai J, Suchartwatnachai C, Jorgensen LN. Effect of combined oral estrogen/progestogen preparation (Kliogest) on bone mineral density, plasma lipids and postmenopausal symptoms in HRT‐naive Thai women. Acta Obstetricia et Gynecologica Scandinavica 2003;82(9):857‐66. - PubMed
Kazerooni 2004 {published data only}
-
- Kazerooni T, Zolghadri J. The comparison of bleeding patterns with high‐dose and low‐dose hormone replacement therapy in postmenopausal women. Gynecological Endocrinology 2004;19(2):64‐8. - PubMed
Keil 2002 {published data only}
-
- Holst TH, Keil D, Lang E. Comparison of the incidence of irregular bleeding during treatment with a low dose continuous combined or sequential hormone replacement therapy. The 1st scientific meeting of the Asia‐Pacific Menopause Federation. 2002; Vol. 02‐05:61.
Limpaphayom 2000 {published data only}
-
- Limpaphayom K, Bunyavejchevin S. Clinical effects of 17 beta‐estradiol and norethisterone acetate in postmenopausal Thai women. Journal of the Medical Association of Thailand 2000;83:407‐15. - PubMed
Liu 2005 {published data only}
-
- Liu JH, Muse KN. The effects of progestins on bone density and bone metabolism in postmenopausal women: a randomized controlled trial. American Journal of Obstetrics & Gynecololgy 2005;192(4):1316‐24. - PubMed
Luciano 1988 {published data only}
-
- Luciano A, Turksoy N, Carleo J, Hendrix J. Clinical and metabolic responses of menopausal women to sequential versus continuous estrogen and progestin replacement therapy. Obstetrics and Gynecology 1988;71:39‐43. - PubMed
Marslew 1991 {published data only}
-
- Marslew U, Riis BJ, Christiansen C. Desogestrel in hormone replacement therapy: long‐term effects on bone, calcium and lipid metabolism, climacteric symptoms, and bleeding. European Journal of Clinical Investigation 1991;21:601‐7. - PubMed
Marslew 1992 {published data only}
-
- Marslew U, Overgaard K, Riis BJ, Christiansen C. Two new combinations of estrogen and progestogen for prevention of postmenopausal bone loss: long‐term effects on bone, calcium and lipid metabolism, climacteric symptoms, and bleeding. Obstetrics and Gynecology 1992;79:202‐10. - PubMed
-
- Marslew U, Riis BJ, Christiansen C. Bleeding patterns during continuous combined estrogen‐progestogen therapy. American Journal of Obstetrics & Gynecology 1991;164(5 Pt 1):1163‐8. - PubMed
Mizunuma 1997 {published data only}
-
- Mizunuma H, Okano H, Soda M, Kagami I, Miyamoto S, Tokizawa T, et al. Prevention of postmenopausal bone loss with minimal uterine bleeding using low dose continuous estrogen/progestin therapy: a 2‐year prospective study. Maturitas 1997;27:69‐76. - PubMed
Morabito 2004 {published data only}
-
- Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, Romeo A, et al. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double‐blind EPT‐ and placebo‐controlled study. Menopause 2004;11(4):400‐4. - PubMed
-
- Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, et al. Effects of genistein and hormone‐replacement therapy on bone loss in early postmenopausal women: a randomized double‐blind placebo‐controlled study. Journal of Bone & Mineral Research 2002;17(10):1904‐12. - PubMed
Nachtigall 1979 {published data only}
-
- Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: A prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstetrics and Gynecology 1979;54(1):74‐9. - PubMed
Odmark 2001 {published data only}
-
- Odmark I, Jonsson B, Backstrom T. Bleeding patterns in postmenopausal women using two types of continuous combined hormone replacement therapy. Gynecological Endocrinology 2000;14(Suppl 2):128.
-
- Odmark IS, Jonsson B, Backstrom T. Bleeding patterns in postmenopausal women using continuous combination hormone replacement therapy with conjugated estrogen and medroxyprogesterone acetate or with 17B‐estradiol and norethindrone acetate. American Journal of Obstetrics and Gynecology 2001;184(6):1131‐8. - PubMed
Pickar 2003a {published data only}
-
- Pickar J, Yeh IT, Cunnane M, Archer D. Impact of conjugated estrogens (CE)/trimegestone (TMG) on endometrial hyperplasia and bleeding profiles in a double‐blind, randomized, placebo‐controlled study. Fertility & Sterility 2003;80(Suppl 3):18.
Pinto 2003 {published data only}
-
- Pinto A, Binder E, Kohrt W, Bronder D, Williams D. Effects of trimonthly progestin administration on the endometrium in elderly postmenopausal women who receive hormone replacement therapy: a pilot study. American Journal of Obstetrics and Gynecology 2003;189:11‐5. - PubMed
Popp 2006 {published data only}
-
- Popp AWE, Bodmer C, Senn C, Fuchs G, Kraenzlin ME, Wyss H, et al. Prevention of postmenopausal bone loss with long‐cycle hormone replacement therapy. Maturitas 2006;53(2):191‐200. - PubMed
Schiff 1982 {published data only}
-
- Schiff I, Sela HK, Cramer D, Tulchinsky D, Ryan KJ. Endometrial hyperplasia in women on cyclic or continuous estrogen regimens. Fertility & Sterility 1982;37(1):79‐82. - PubMed
Simon 2001 {published data only}
-
- Simon J, Symons J. Unscheduled bleeding during initiation of continuous combined hormone replacement therapy:a direct comparison of two combinations of norethindrone acetate and ethinyl estradiol to medroxyprogesterone acetate and conjugated equine estrogens. Menopause 2001;8:321‐6. - PubMed
Simon 2003 {published data only}
-
- Simon J, Liu J, Speroff L, Speroff L, Shumel B, Symons J. Reduced vaginal bleeding in postmenopausal women who receive combined norethindrone acetate and low‐dose ethinyl estradiol therapy versus combined conjugated equine estrogens and medroxyprogesterone acetate therapy. American Journal of Obstetrics & Gynecology 2003;188:92‐9. - PubMed
Steiner 2007 {published data only}
-
- Steiner AZ, Xiang M. Unopposed estradiol therapy in postmenopausal women: results from two randomized trials. Obstetrics & Gynecology 2007;109(3):581‐7. - PubMed
Stevenson 2001 {published data only}
-
- Stevenson JC, Teter P, Lees B. 17beta‐Estradiol (1 mg/day) continuously combined with dydrogesterone (5, 10 or 20 mg/day) increases bone mineral density in postmenopausal women. Maturitas 2001;38(2):197‐203. - PubMed
Stevenson 2010 {published data only}
-
- Stevenson Jc DGKEPT. Oral ultra‐low dose continuous combined hormone replacement therapy with 0.5 mg 17[beta]‐oestradiol and 2.5 mg dydrogesterone for the treatment of vasomotor symptoms: results from a double‐blind, controlled study. Maturitas 2010; Vol. 67, issue 3:227‐32. - PubMed
Sturdee 1996 {published data only}
-
- Sturdee DW. Bleeding patterns and endometrial histology with sequential HRT. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:S36.
Sturdee 2000 {published data only}
-
- Sturdee DW, Ulrich LG, Barlow DH, Wells M, Campbell MJ, Vessey MP, et al. The endometrial response to sequential and continuous combined oestrogen‐progestogen replacement therapy. British Journal of Obstetrics and Gynecology 2000;11:1392‐400. - PubMed
Sturdee 2008 {published data only}
-
- Sturdee DW ADFRVLECSI. Ultra‐low‐dose continuous combined estradiol and norethisterone acetate: improved bleeding profile in postmenopausal women. Climacteric : the journal of the International Menopause Society 2008; Vol. 11, issue 1:63‐73. - PubMed
Symons 2000 {published data only}
-
- Symons J, Kempfert N, Speroff L. Vaginal bleeding in postmenopausal women taking low dose norethindrone acetate and ethinyl estradiol combinations. Obstetrics & Gynecology 2000;96:366‐72. - PubMed
Symons 2002 {published data only}
-
- Symons J. Comparative effect of norethindrone acetate/ethinyl estradiol and 0.625 mg conjugated estrogen/2.5 mg medroxyprogesterone acetate on bleeding control: early results from a randomized placebo controlled trial.. Obstetrics & Gynecology 2002;Suppl:84.
Ulla Timonen 2002 {published data only}
-
- Ulla Timonen R, Heikkinen R, Vaheri P, Kainulainen P. Bleeding control, endometrial safety and adherence to continuous combined HRT after 5 years. Climacteric 2002;P‐07‐21:146.
Utian 2002 {published data only}
-
- Utian W, Leonard T, Davis A, Vega R. Efficacy and safety study of a new synthetic 10‐component, modified release conjugated estrogens (CE) tablet for treatment of vasomotor symptoms in postmenopausal women. Fertility & Sterility 2002;78(3 Suppl 1):S159.
van der Mooren 1996 {published data only}
-
- Mooren MJ, Hanselaar A, Schiff Ch PT, Girardin C. Sequential three‐monthly versus monthly HRT: a prospective double‐blind randomised study. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:47.
van de Weijer 2002 {published data only}
-
- Weijer P. Long‐term data on endometrial safety and bleeding control with a continuous combined HRT regimen. Climacteric. 10th World Congress on Menopause 2002;5(Suppl 1):210.
Veerus 2008 {published data only}
Volpe 1986 {published data only}
-
- Volpe A, Facchinetti F, Grasso A, Petraglia F, Campanini D, Genazzani AR. Benefits and risks of different hormonal replacement therapies in post‐menopausal women. Maturitas 1986;6:327‐34. - PubMed
Von Holst 2002 {published data only}
-
- Holst T, Lang E, Winkler U, Keil D. Bleeding patterns in peri and postmenopausal women taking a continuous combined regimen of estradiol with norethisterone acetate or a conventional sequential regimen of conjugated equine estrogens with medrogestone. Maturitas 2002;43(4):265‐75. - PubMed
Wahab 2002 {published data only}
-
- Wahab M, Thompson J, Whitehead M, Al‐Azzawi F. The effect of a change in the dose of trimegestone on the pattern of bleeding in estrogen‐treated post‐menopausal women: 6 month extension of a dose‐ranging study. Human Reproduction 2002;17(5):1386‐90. - PubMed
Wang 2006 {published data only}
-
- Wang SH, Lin SQ, Gui QF, Jin MJ, Jiang Y. Profiles of irregular bleeding induced by low‐dose hormone therapy and Chinese formulated herb products. Acta Academiae Medicinae Sinicae 2006;28(2):256‐61. - PubMed
Warming 2004a {published data only}
-
- Warming L, Ravn P, Spielman D, Delmas P, Christiansen C. Trimegestone in a low‐dose, continuous‐combined hormone therapy regimen prevents bone loss in osteopenic postmenopausal women. Menopause 2004;11(3):337‐42. - PubMed
Webster 1996 {published data only}
-
- Webster M. Combination continuous therapy ‐ what is the optimal MPA dose? Effect on bleeding profiles and endometrial histology. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:27.
Weinstein 1990 {published data only}
-
- Weinstein LB. Evaluation of a continuous combined low‐dose regimen of estrogen‐progestin for treatment of the menopausal patient. American Journal of Obstetrics & Gynecology 1990;162(6):1534‐9. - PubMed
Wells 2002 {published data only}
Williams 1990 {published data only}
-
- Williams SR, Frenchek B, Speroff T, Speroff L. A study of combined continuous ethinyl estradiol and norethindrone acetate for postmenopausal hormone replacement. American Journal of Obstetrics and Gynecology 1990;162:438‐46. - PubMed
Yang 2001 {published data only}
-
- Yang TS, Liang WH, Chang SP, Yuan CC. Effects of period free hormone replacement therapy in postmenopausal women in Taiwan. Chinese Medical Journal (Taipei) 2001;65:23‐8. - PubMed
Additional references
Ansbacher 1994
-
- Ansbacher R. Estrogens; conjugated and esterified therapeutic substitution. Female Pat 1994;19:14‐20.
Antunes 1979
-
- Antunes CM, Strolley PD, Rosenshein NB, Davies JL, Tonascia JA, Brown C, Burnett L, et al. Endometrial cancer and estrogen use. Report of a large case‐control study. New England Journal of Medicine 1979;300(1):9‐13. - PubMed
Archer 1999a
-
- Archer DF, Dorin MH, Heine W, Nayan N, Arce JC. Uterine bleeding in postmenopausal women on continuous therapy with oestradiol and norethindrone acetate. Obstetrics & Gynecology 1999;94(3):323‐9. - PubMed
Bachmann 2007
-
- Bachmann GA, Schaefers M, Uddin A, Utian WH. Lowest effective transdermal 17beta‐estradiol dose for relief of hot flushes in postmenopausal women: a randomized controlled trial. Obstetrics & Gynecology 2007; Vol. 110, issue 4:771‐9. [0029‐7844: (Print)] - PubMed
Cust 1990
-
- Cust MP, Gangar KF, Hillard TC, Whitehead MI. A risk benefit assessment of oestrogen therapy in post menopausal women. Drug Safety 1990;5:345‐358. - PubMed
Ellerington 1992
-
- Ellerington MC, Whitecroft SIV, Whitehead MI. HRT: Developments in therapy. British Medical Bulletin 1992;48:401‐25. - PubMed
France 1998
-
- France J. Personal communication July 1998.
Freeman 2011
Fugh‐Berman 2010
Gardan 1977
-
- Gardan J, Reagan JW, Finkle WD. Oestrogen and endometrial carcinoma: an independent pathology review supporting original size estimate. New England Journal of Medicine 1977;297:570. - PubMed
Grady 1995
-
- Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta‐analysis. Obstetrics & Gynecology 1995;85:304‐13. - PubMed
Hickey 2005
-
- Hickey M, Davis SR, Sturdee DW. Treatment of menopausal symptoms: what shall we do now?. Lancet 2005;366(9483):409‐21. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kumar 2000
-
- Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids 2000;65(10‐1):629‐36. - PubMed
Kurman 1985
-
- Kurman RJ, Kaminshi PF, Norm HJ. The behaviour of endometrial hyperplasia. A long term study of 'untreated' hyperplasia in 170 patients. Cancer 1985;56:403‐12. - PubMed
Lawrie 2011
Mack 1976
-
- Mack TM, Pike MC, Henderson BE, Pfeffer RI, Gerkins VR, Arthur M, et al. Estrogens and endometrial cancer in a retirement community. New England Journal of Medicine 1976;294:1262‐7. - PubMed
MacLennan 1998
-
- MacLennan AH. Personal communication 16 March 1998; 13 August 1998.
Nelson 2002
-
- Nelson H, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy. JAMA 2002;288(7):872‐81. - PubMed
O'Connell 1998
-
- O'Connell D, Robertson J, Henry D, Gillespie W. A systematic review of the skeletal effects of estrogen therapy in postmenopausal women. II An assessment of treatment effects. Climacteric 1998;1:112‐23. - PubMed
Peeyananjarassri 2005
-
- Peeyananjarassri K, Baber R. Effects of low‐dose hormone therapy on menopausal symptoms, bone mineral density, endometrium, and the cardiovascular system: a review of randomised clinical trials. Climacteric 2005;8(1):13‐23. - PubMed
Pickar 2003
-
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two‐year substudy results. Fertility & Sterility 2003;80(5):1234‐40. - PubMed
Pike 1997
-
- Pike MC, Peters RK, Cozen W, Probst‐Hensch NM, Felix JC, Wan PC, et al. Estrogen‐progestin replacement therapy and endometrial cancer. Journal of the National Cancer Institute 1997;89:1110‐6. - PubMed
Politi 2008
Raudaskoski 2002
-
- Raudaskoski T, Tapanainen J, Tomas E, Luotola H, Pekonen F, Ronni‐Sivula, H, et al. Intrauterine 10mug and 20mug levonorgestrel systems in postmenopausal women receiving oral oestrogen replacement therapy: Clinical, endometrial and metabolic response. BJOG: An International Journal of Obstetrics & Gynaecology 2002;109(2):136‐44. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Smith 1977
-
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous oestrogen and endometrial carcinoma. New England Journal of Medicine 1977;293:1164‐7. - PubMed
Speroff 2005
-
- Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility (7th edition). Lippincott Williams & Wilkins & Wilkins, 2005.
Sturdee 1994
-
- Sturdee DW, Barlow DH, Ulrich LG, Wells M, Gydesen H, Campbell M, et al. Is the timing of withdrawal bleeding a guide to endometrial safety during sequential oestrogen‐progestogen replacement therapy?. Lancet 1994;344(8928):979‐82. - PubMed
Terakawa 1997
-
- Terakawa N, Kigawa J, Taketani Y, Yoshikawa H, Yajima A, Noda K. The behaviour of endometrial hyperplasia: a prospective study. Journal of Obstetrics and Gynaecology Research 1997;28:223‐30. - PubMed
Udoff 1995
-
- Udoff L, Langenberg P, Adashi E. Combined continuous hormone replacement therapy: a clinical review. Obstetrics & Gynecology 1995;86:306‐16. - PubMed
Utian 2001
-
- Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertility & Sterility 2001;75(6):1065‐79. - PubMed
Whitehead 1977
-
- Whitehead MI, Queen J, Beard RN, Minardi J, Campbell S. The effects of cyclical oestrogen therapy and sequential oestrogen therapy on the endometrium of postmenopausal women. Acta Obstetrica et Gynecologica Scandinavica Supplement 1977;65:91‐101. - PubMed
Whitehead 1981
-
- Whitehead MI, Townsend PT, Pryse‐Davies J, Ryder TA, King RJB. Effects of estrogens and progestins on the biochemistry and morphology of the postmenopausal endometrium. New England Journal of Medicine 1981;305:1599‐605. - PubMed
Whitehead 1987
-
- Whitehead MI, Fraser D. Controversies concerning the safety of estrogen replacement therapy. American Journal of Obstetrics & Gynecology 1987;156:13113‐22. - PubMed
Wood 2008
Ziel 1975
-
- Ziel HK, Finkle WD. Increased risk of endometrial carcinoma amongst users of conjugated oestrogens. New England Journal of Medicine 1975;293:1167‐70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

