Prostaglandins for preventing postpartum haemorrhage
- PMID: 22895917
- PMCID: PMC7043277
- DOI: 10.1002/14651858.CD000494.pub4
Prostaglandins for preventing postpartum haemorrhage
Abstract
Background: Prostaglandins have mainly been used for postpartum haemorrhage (PPH) when other measures fail. Misoprostol, a new and inexpensive prostaglandin E1 analogue, has been suggested as an alternative for routine management of the third stage of labour.
Objectives: To assess the effects of prophylactic prostaglandin use in the third stage of labour.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (7 January 2011). We updated this search on 25 May 2012 and added the results to the awaiting classification section.
Selection criteria: Randomised trials comparing a prostaglandin agent with another uterotonic or no prophylactic uterotonic (nothing or placebo) as part of management of the third stage of labour. The primary outcomes were blood loss 1000 mL or more and the use of additional uterotonics.
Data collection and analysis: Two review authors independently assessed eligibility and trial quality and extracted data.
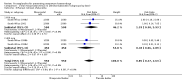
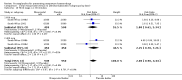
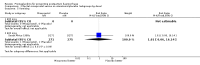
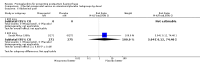
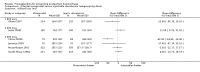
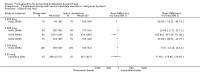
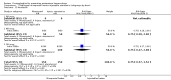
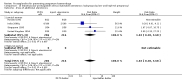
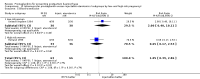
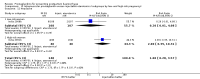
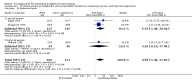
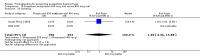
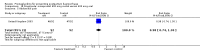
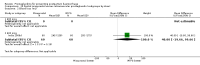
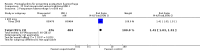
Main results: We included 72 trials (52,678 women). Oral or sublingual misoprostol compared with placebo is effective in reducing severe PPH (oral: seven trials, 6225 women, not totalled due to significant heterogeneity; sublingual: risk ratio (RR) 0.66; 95% confidence interval (CI) 0.45 to 0.98; one trial, 661 women) and blood transfusion (oral: RR 0.31; 95% CI 0.10 to 0.94; four trials, 3519 women).Compared with conventional injectable uterotonics, oral misoprostol was associated with higher risk of severe PPH (RR 1.33; 95% CI 1.16 to 1.52; 17 trials, 29,797 women) and use of additional uterotonics, but with a trend to fewer blood transfusions (RR 0.84; 95% CI 0.66 to 1.06; 15 trials; 28,213 women). Additional uterotonic data were not totalled due to heterogeneity. Misoprostol use is associated with significant increases in shivering and a temperature of 38º Celsius compared with both placebo and other uterotonics.
Authors' conclusions: Oral or sublingual misoprostol shows promising results when compared with placebo in reducing blood loss after delivery. The margin of benefit may be affected by whether other components of the management of the third stage of labour are used or not. As side-effects are dose-related, research should be directed towards establishing the lowest effective dose for routine use, and the optimal route of administration.Neither intramuscular prostaglandins nor misoprostol are preferable to conventional injectable uterotonics as part of the management of the third stage of labour especially for low-risk women; however, evidence has been building for the use of oral misoprostol to be effective and safe in areas with low access to facilities and skilled healthcare providers and future research on misoprostol use in the community should focus on implementation issues.
Conflict of interest statement
Two review authors (AM Gülmezoglu, GJ Hofmeyr) participated in the WHO 1999 and WHO 2001 trials and one review author (GJ Hofmeyr) participated in Africa 2011; South Africa 1998a; South Africa 1998b; South Africa 1998c; South Africa 1998d. GJ Hofmeyr did not assess the trials or extract the data for the trials in which he was involved. AM Gülmezoglu extracted the data but these were independently checked by Fatu Forna, an author on the earlier version of the review.
Figures









































































































































































































Update of
-
Prostaglandins for preventing postpartum haemorrhage.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD000494. doi: 10.1002/14651858.CD000494.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD000494. doi: 10.1002/14651858.CD000494.pub4. PMID: 17636640 Updated.
References
References to studies included in this review
Africa 2011 {published data only}
-
- Hofmeyr GJ. Misoprostol for preventing postpartum hemorrhage. ClinicalTrials.gov (www.clinicaltrials.gov) (accessed 20 February 2008).
-
- Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, et al. Administration of 400mug of misoprostol to augment routine active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2011;112(2):98‐102. - PubMed
Australia 1999 {published data only}
-
- Cook C, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or syntometrine in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(4):414‐9. - PubMed
Bangladesh 2007 {published data only}
-
- Sultana N, Khatun M. Misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Bangladesh College of Physicians and Surgeons 2007;25(2):73‐6.
Belgium 1999 {published data only}
-
- Amant F, Spitz B, Timmerman D, Corremans A, Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double‐blind randomised trial. British Journal of Obstetrics and Gynaecology 1999;106:1066‐70. - PubMed
Canada 2002 {published data only}
-
- Karkanis S, Caloia D, Salenieks M, Kingdom J, Walker M, Meffe F, et al. Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. Journal of Obstetrics & Gynaecology of Canada: JOGC 2002;24(2):149‐54. - PubMed
Canada 2005 {published and unpublished data}
-
- Baskett TF, Persad V, Clough H, Young D. Prophylactic use of misoprostol in the third stage of labor [abstract]. Obstetrics & Gynecology 2005;105(4 Suppl):39S.
-
- Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. International Journal of Gynecology & Obstetrics 2007;97(1):2‐5. - PubMed
China 2003a {published data only}
-
- Fu YX, Ran KQ, Wang M. Prevention of early postpartum hemorrhage by way of oral misoprostol. Journal of Nursing Science 2003;18(12):910‐1.
China 2003b {published data only}
-
- Li D, Bei HZ. Clinical study on reduction of postpartum bleeding in the risk factors by misoprostol. Hainan Medical Journal 2003;14(11):11‐2.
China 2004a {published data only}
-
- Lam H, Tang OS, Lee CP, Ho PC. A pilot‐randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:647‐50. - PubMed
China 2007 {published data only}
-
- Ng PS, Lai CY, Sahota DS, Yuen PM. A double‐blind randomized controlled trial of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor. Gynecologic and Obstetric Investigation 2007;63(1):55‐60. - PubMed
Colombia 2002 {published data only}
-
- Penaranda W, Arrieta O, Yances B. Active management of the childbirth with sublingual misoprostol: a clinical controlled trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en el Hospital de Maternidad Rafael Calvo de Cartagena]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87‐92.
Egypt 1993 {published data only}
-
- Abdel‐Aleem H, Abol‐Oyoun EM, Moustafa SAM, Kamel HS, Abdel‐Wahab HA. Carboprost trometamol in the management of the third stage of labor. International Journal of Gynecology & Obstetrics 1993;42:247‐50. - PubMed
Egypt 1997 {unpublished data only}
-
- Abdel‐Aleem H, Mostafa SAM, Makarem MH, Abol‐Oyoun EM, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum hemorrhage. Assiut Medical Journal 1994;18:15.
-
- Abdel‐Aleem H, Mostafa SAM, Makarem MH, Abol‐Oyoun EM, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum hemorrhage. The First World Congress on Maternal Mortality; 1997 March 8‐14; Morocco. 1997.
Egypt 2009 {published data only}
-
- Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;105(3):244‐7. - PubMed
France 2001 {published data only}
-
- Benchimol M, Gondry J, Mention J, Gagneur O, Boulanger J. Role of misoprostol in controlled delivery [Place du misoprostol dans la direction de la delivrance]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(6):576‐83. - PubMed
Gambia 2005 {published data only}
-
- Walraven G, Blum J, Dampha Y, Sowe M, Morison L, Winikoff B, et al. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112(9):1277‐83. - PubMed
Ghana 2000 {published data only}
-
- Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double‐blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1111‐5. - PubMed
Ghana 2006 {published data only}
-
- Parsons S, Ntumy YM, Walley RL, Wilson JB, Crane JMG, Matthews K, et al. Rectal misoprostol vs intramuscular oxytocin in the routine management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9 Glasgow, UK. 2004:18.
-
- Parsons S, Walley RL, Crane JMG, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics & Gynaecology of Canada: JOGC 2006;28:20‐6. - PubMed
Ghana 2007 {published data only}
-
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2007;29(9):711‐8. - PubMed
Guinea‐Bissau 2005 {published data only}
-
- Nielsen BB, Hoj L, Hvidman LE, Nielsen J, Cardoso P, Aaby P. Reduced post‐partum bleeding after treatment with sublingual misoprostol: a randomized double‐blind clinical study in a developing country ‐ secondary publication. Ugeskrift for Laeger 2006;168(13):1341‐3. - PubMed
Holland 1991 {published data only}
-
- Poeschmann RP, Doesburg WH, Eskes TKAB. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1991;98:528‐30. - PubMed
-
- Poeschmann RP, Eskes TKAB, Doesburg WH. Oxytocin and sulprostone reduce postpartum blood los and shorten the third stage in low risk term women. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15‐20; Singapore. 1991:312.
-
- Poeschmann RP, Eskes TKAB, Doesburg WH, Lemmens WAJG, Benneker JCLH. Oxytocin and sulprostone reduce postpartum blood loss in low risk term women compared to saline. Proceedings of the 1st European Congress on Prostaglandins in Reproduction (ECPR); 1988 July 6‐9; Vienna, Austria. 1988:176.
Holland 1995 {published data only}
-
- Selm M, Kanhai HHH, Keirse MJNC. Preventing the recurrence of atonic postpartum hemorrhage: a double blind trial. Acta Obstetricia et Gynecologica Scandinavica 1995;74:270‐4. - PubMed
Hong Kong 2001 {published data only}
-
- Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and i.m. syntometrine in the management of the third stage of labour. Human Reproduction 2001;16(1):31‐5. - PubMed
-
- Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. Comparison of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor a multicenter randomised controlled trial. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29.
India 1988c {published data only}
-
- Bhattacharya P, Devi PK, Jain S, Kanthamani CR, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route for control of postpartum bleeding ‐ a comparative trial with methylergometrine. Acta Obstetricia et Gynecologica Scandinavica Supplement 1988;145:13‐5. - PubMed
India 2001b {published data only}
-
- Reddy R, Shenoy JV. Active management of third stage of labour. A comparative study in high risk patients for atonic postpartum haemorrhage. Journal of Obstetrics and Gynecology of India 2001;51(2):44‐7.
India 2004b {published data only}
-
- Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methlergometrine for active management of third stage of labor. International Journal of Gynecology & Obstetrics 2004;87:1‐5. - PubMed
India 2005a {published data only}
-
- Garg P, Batra S, Gandhi G. Oral misoprostol versus injectable methyergometrine in management of the third stage of labour. International Journal of Gynecology & Obstetrics 2005;91:160‐1. - PubMed
India 2006a {published data only}
-
- Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. International Journal of Gynecology & Obstetrics 2006;92:106‐10. - PubMed
India 2006b {published data only}
-
- Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labour. International Journal of Gynecology & Obstetrics 2006;92:23‐6. - PubMed
India 2006c {published and unpublished data}
-
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum hemorrhage in a community setting. International Conference. Evidence‐based interventions to prevent postpartum haemorrhage: translating research into practice; 2006 July 13‐14; Goa, India. 2006.
-
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum hemorrhage in a community setting. Lancet 2006;368(9543):1248‐53. - PubMed
-
- Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, et al. Conducting international collaborative research in developing nations. International Journal of Gynecology & Obstetrics 2004;87(3):267‐71. - PubMed
-
- Goudar SS, Chakraborty H, Edlavitch SA, Naik VA, Bellad MB, Patted SS, et al. Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. Journal of Maternal‐Fetal & Neonatal Medicine 2008;21(8):559‐64. - PubMed
India 2006d {published data only}
-
- Nellore V, Mittal S, Dadhwal V. Rectal misoprostol vs. 15‐methyl prostaglandin F2‐alpha for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94:45‐6. - PubMed
India 2006f {published data only}
-
- Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage ‐ a pilot study. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S139‐S140. - PubMed
India 2006g {published data only}
-
- Nagaria T, Ekka M. Intramuscular PGF2 a 125 microg versus intravenous methyl ergometrine 0.2mg in the active management of third stage of labor. Journal of Obstetrics and Gynaecology of India 2006;56(4):396‐8.
India 2007 {published data only}
-
- Biswas A, Bal R, Kundu MK, Kyal A, Halder M. A study of prophylactic use of 15‐methyl prostaglandin f2alpha in the active management of third stage of labour. Journal of the Indian Medical Association 2007;105(9):506, 508‐9. - PubMed
India 2008a {published data only}
-
- Chhabra S, Tickoo C. Low‐dose sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 2008;34(5):820‐3. - PubMed
India 2008b {published and unpublished data}
-
- Kushtagi P, Verghese LM. Evaluation of two uterotonic medications for the management of the third stage of labor. International Journal of Gynecology & Obstetrics 2006;94(1):47‐8. - PubMed
-
- Verghese L, Kushtagi P. Evaluation of carboprost as a prophylactic oxytocic in the management of third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):74.
India 2009b {published data only}
-
- Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2009;107(2):130‐4. - PubMed
India 2009d {published data only}
-
- Vaid A, Dadhwal V, Mittal S, Deka D, Misra R, Sharma JB, et al. A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl‐ergometrine versus intramuscular 15‐methyl PGF2alpha in active management of third stage of labor. Archives of Gynecology and Obstetrics 2009;280(6):893‐7. - PubMed
India 2010 {published data only}
-
- Khurshid R, Fatima K, Parveen S, Ul Shamas I, Salman R. A comparison between intramuscular PGF2 a125 mG and intravenous methyl ergometrine 0.2 Mg in the active management of third stage labor. Internet Journal of Gynecology and Obstetrics 2010;14(1):1‐6.
Iran 2009 {published data only}
-
- Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. Journal of Obstetrics and Gynaecology 2009;29(7):633‐6. - PubMed
Jamaica 2009 {published data only}
-
- Harriott J, Christie L, Wynter S, DaCosta V, Fletcher H, Reid M. A randomized comparison of rectal misoprostol with syntometrine on blood loss in the third stage of labour. West Indian Medical Journal 2009;58(3):201‐6. - PubMed
Mexico 2009 {published data only}
-
- Quiroga Diaz R, Cantu Mata R, Tello Gutierrez HE, Puente Villalobos M, Montemayor Garza R, Martinez Mendoza A. Intrauterine misoprostol for the prevention of bleeding cesarean. Ginecologia y Obstetricia de Mexico 2009;77(10):469‐74. - PubMed
Mozambique 2001 {published data only}
-
- Bugalho A, Daniel A, Faundes A, Cunha M. Misoprostol for prevention of postpartum haemorrhage. International Journal of Gynecology & Obstetrics 2001;73:1‐6. - PubMed
Nigeria 2003 {published data only}
-
- Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Obstetrics and Gynaecology 2003;23:13‐6. - PubMed
Nigeria 2007 {published data only}
-
- Enakpene CA, Morhason‐Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post‐partum hemorrhage during third stage of labor. Journal of Obstetrics and Gynaecology Research 2007;33(6):810‐7. - PubMed
Nigeria 2011 {published data only}
-
- Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al. A double‐blind, randomized, placebo‐controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2011;112(2):107‐11. - PubMed
Pakistan 2011 {published data only}
-
- Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum hemorrhage in homebirths in Pakistan: a randomised placebo controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353‐61.. - PMC - PubMed
-
- Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Use of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage during home deliveries in Pakistan: a randomised placebo‐controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S92. - PMC - PubMed
-
- Mobeen N, Walraven G. Misoprostol for the prevention of postpartum hemorrhage in rural Pakistan (ongoing trial). www.clinicaltrials.gov (accessed 21 March 2006).
Singapore 1995 {published data only}
-
- Chua S, Chew SL, Yeoh CL, Roy AC, Ho LM, Selamat N, et al. A randomized controlled study of prostaglandin 15‐methyl F2alpha compared with syntometrine for prophylactic use in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1995;35(4):413‐6. - PubMed
South Africa 1998a {published data only}
-
- Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. Randomized comparison of rectal misoprostol with syntometrine for management of third stage of labor. Acta Obstetricia et Gynaecologica Scandinavica 1998;77:178‐81. - PubMed
South Africa 1998b {published data only}
-
- Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105:971‐5. - PubMed
-
- Hofmeyr GJ, Jager M, Rose L, Nikodem VC, Lawrie T. Misoprostol for third stage of labour management: a double blind, placebo controlled clinical trial. Proceedings of the 16th Conference on Priorities in Perinatal Care in South Africa; 1997; South Africa. 1997:29‐31.
South Africa 1998c {published data only}
-
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum haemorrhage: a placebo controlled trial. Proceedings of the 17th Conference on Priorities in Perinatal Care in South Africa; 1998; South Africa. 1998:49‐52. - PubMed
-
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1998;179(4):1043‐6. - PubMed
South Africa 1998d {published and unpublished data}
-
- Hofmeyr GJ, Nikodem C, Jager M, Drakely A, Gilbart B. Oral misoprostol for labour third stage management: randomised assessment of side effects (part 2). Proceedings of the 17th Conference on Priorities in Perinatal Care; 1988; South Africa. 1998:53‐4.
South Africa 2001 {published and unpublished data}
-
- Hofmeyr G, Nikodem V, Jager M, Drakely A. Side effects of oral misoprostol in the third stage of labour: a random allocation placebo controlled trial. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S40‐S41.
-
- Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Oral misoprostol for labour third stage management: randomised assessment of side effects (part 1). Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:53‐4.
-
- Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Side‐effects of oral misoprostol in the third stage of labour: a randomised placebo controlled trial. South African Medical Journal 2001;91(5):432‐5. - PubMed
Switzerland 1999 {published data only}
-
- Surbek DV, Fehr P, Hoesli I, Holzgreve W. Oral misoprostol for third stage of labor: a randomized placebo‐controlled trial. Obstetrics & Gynecology 1999;94:255‐8. - PubMed
-
- Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Oral misoprostol vs placebo for third stage of labor [Orales misoprostol reduziert den postpartalen blutverlust]. Gynakologisch Geburtshilfliche Rundschau 1999;39:144.
Switzerland 2006 {published data only}
-
- Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. International Journal of Gynecology & Obstetrics 2006;95(1):2‐7. - PubMed
Tibet 2009 {published data only}
-
- Tudor C, Miller S, Nyima, Sonam, Droyoung, Varner M. Preliminary progress report: randomized double‐blind trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum hemorrhage in Lhasa, Tibet. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S145‐S146. - PubMed
-
- Wright L. Preventing postpartum hemorrhage using a Tibetan traditional medicine. www.clinicaltrials.gov (accessed 21 March 2006).
Tunisia 2009 {published data only}
-
- Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al. Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2009;38(7):588‐93. - PubMed
Turkey 2002 {published data only}
-
- Caliskan E, Meydanli M, Dilbaz B, Aykan B, Sonmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2002;187:1038‐45. - PubMed
Turkey 2003 {published data only}
-
- Caliskan E, Dilbaz B, Meydanli M, Ozturk N, Narin M, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2003;101:921‐8. - PubMed
Turkey 2010 {published data only}
-
- Ozalp E, Tanir HM, Sener T. Dinoprostone vaginal insert versus intravenous oxytocin to reduce postpartum blood loss following vaginal or cesarean delivery. Clinical and Experimental Obstetrics and Gynecology 2010;37(1):53‐5. - PubMed
-
- Tanir H, Sener T, Ozalp E. Dinoprostone vaginal insert versus intravenous oxytocin to reduce the postpartum blood loss following vaginal or cesarean delivery. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S506‐S507. - PubMed
United Kingdom 1994 {published data only}
-
- Chou MM, MacKenzie IZ. A prospective, double blind, randomized comparison of prophylactic intramyometrial 15‐methyl prostaglandin F2alpha, 125 micrograms, and intravenous oxytocin, 20 units, for the control of blood loss at elective cesarean section. American Journal of Obstetrics and Gynecology 1994;171:1356‐60. - PubMed
United Kingdom 2000 {unpublished data only}
-
- El‐Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, et al. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG: an international journal of obstetrics and gynaecology 2000;107:1104‐10. - PubMed
United Kingdom 2001b {published data only}
-
- Lokugamage A, Paine M, Bassaw‐Balroop K, Sullivan K, el‐Refaey H, Rodeck C. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(4):411‐4. - PubMed
-
- Lokugamage A, Paine M, Bassaw‐Balroop K, Sullivan K, el‐Refaey H, Rodeck C. Active management of the third stage at caesarean section: misoprostol vs syntocinon. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 2). 2000:54.
United Kingdom 2001c {published data only}
-
- Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins & Other Lipid Mediators 2001;66(3):203‐10. - PubMed
United Kingdom 2003 {published data only}
-
- Khan R, El‐Refaey H. Pharmacokinetics and adverse‐effect profile of rectally administered misoprostol in the third stage of labor. Obstetrics & Gynecology 2003;101(5):968‐74. - PubMed
USA 1990 {published data only}
-
- Catanzarite VA. Prophylactic intramyometrial carboprost tromethamine does not substantially reduce blood loss relative to intramyometrial oxytocin at routine cesarean section. American Journal of Perinatology 1990;7:39‐42. - PubMed
USA 2001 {published data only}
-
- Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185:878‐82. - PubMed
USA 2004 {published data only}
-
- Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstetrics & Gynecology 2004;104(6):1282‐8. - PubMed
USA 2005 {published data only}
-
- Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. American Journal of Obstetrics and Gynecology 2005;192:1404‐6. - PubMed
WHO 1999 {published and unpublished data}
-
- Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose‐related shivering and pyrexia in the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106:304‐8. - PubMed
WHO 2001 {published and unpublished data}
-
- Gülmezoglu AM, Villar J, Ngoc NN, Piaggio G, Carroli G, Adetoro L, et al. The WHO multicentre double‐blind randomized controlled trial to evaluate the use of misoprostol in the management of the third stage of labour. Lancet 2001;358(9283):689‐95. - PubMed
-
- Lumbiganon P, Villar J, Piaggio G, Gülmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:1222‐6. - PubMed
Zimbabwe 2001 {published data only}
-
- Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2001;75:235‐41. - PubMed
References to studies excluded from this review
Australia 2009 {published data only}
-
- Dickinson JE, Doherty DA. Optimization of third‐stage management after second‐trimester medical pregnancy termination. American Journal of Obstetrics & Gynecology 2009;201(3):303.e1‐303.e7. - PubMed
Austria 1983 {published data only}
-
- Baumgarten K, Schmidt J, Horvat A, Neumann M, Cerwenka R, Gruber W, et al. Uterine motility after post‐partum application of sulprostone and other oxytocics. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;16:181‐92. - PubMed
Canada 2004 {published data only}
-
- Chandra S, Persad V, Young D, Baskett T. A preliminary study of cutaneous blood flow associated with postpartum use of oral misoprostol. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(12):1073‐6. - PubMed
China 1997 {published data only}
-
- Liu C, Wang D, Li X, Yuxia Y, Jian Z, Tao L. Clinical study on reduction of postpartum bleeding by methyl carprost suppository. Chinese Journal of Obstetrics and Gynecology 1997;32(1):22‐4. - PubMed
China 1998 {published data only}
-
- Zhao Y, Li X, Peng Y. Clinical study on reduction of postpartum bleeding in cesarean section by misoprostol. Chung‐Hua Fu Chan Ko Tsa Chih (Chinese Journal of Obstetrics and Gynecology) 1998;33(7):403‐5. - PubMed
China 1998b {published data only}
-
- Weihong H, Hanrong L, Linan C. Preventing of postpartum haemorrhage by Carboprost Methylate suppository administered through vagina or sublingually. Acta Academiae Medicinae Shangai 1998;25(2):137‐9.
China 2000 {published data only}
-
- Jin LJ, Zhou L. Application of anus misoprostol to decrease the volume of post partum hemorrhage. Journal of Practical Nursing 2000;16(2):9‐10.
China 2001 {published data only}
-
- Jiang Q, Wang P, Cao W. Effect on different doses of misoprostol to prevent postpartum hemorrhage. Chinese Nursing Research 2001;15(6):313‐4.
China 2002 {published data only}
-
- Li X, Wang H, Wang J, Cao X L, Ma Y. Prophylactic and therapeutic effect of misoprofil plus oxytocin on postpartum hemorrhage in patients with pregnancy‐induced hypertension syndrome. Journal of Postgraduates of Medicine 2002;25(7):34‐5.
China 2003c {published data only}
-
- Xu H. Misoprostol on preventing postpartum bleeding in cesarean. Hebei Medicine 2003;9(9):806‐7.
China 2003d {published data only}
-
- Zhao SF, Sun XF. Clinical study on preventing and curing postpartum hemorrhage in the third stage of labor. Journal of Practical Obstetrics and Gynecology 2003; Vol. 19, issue 5:278‐80.
China 2004b {published data only}
-
- Ng PS, Yuen PM, Sahota DS. Comparison of oral misoprostol and intravascular syntocinon in the management of the third stage of labour ‐ a double‐blind randomised controlled trial. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9 Glasgow, UK. 2004:69.
China 2004c {published data only}
-
- Yuen PM, Ng PS, Sahota DS. A double‐blind randomised controlled trial of oral misoprostol in addition to intra‐muscular syntometrine in the management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9 Glasgow, UK. 2004:62.
Egypt 1999 {published data only}
-
- Diab KM, Ramy AR, Yehia MA. The use of rectal misoprostol as active pharmacological management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 1999;25(5):327‐32. - PubMed
Hungary 1979 {published data only}
-
- Kerekes L, Domokos N. The effect of prostaglandin F2alpha on third stage of labour. Prostaglandins 1979;18:161‐6. - PubMed
India 1988a {published data only}
-
- Devi PK, Sutaria UD, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha for control of postpartum bleeding. Acta Obstetricia et Gynaecologica Scandinavica Supplement 1988;145:7‐8. - PubMed
India 1988b {published data only}
-
- Anjaneyulu R, Devi PK, Jain S, Kanthamani CR, Vijaya R, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route ‐ a controlled clinical trial. Acta Obstetricia et Gynecologica Scandinavica Supplement 1988;145:9‐11. - PubMed
India 2000a {published data only}
-
- Chatterjee A. Misoprostol and the third stage. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29.
India 2000b {published data only}
-
- Dutta DK, Saha KK. Comparative study on role of syntometrine and prostaglandin in the prevention of PPH. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29.
India 2000c {published data only}
-
- Rajwani J, Survana K. Active management of third stage of labor ‐ a comparative study. XVI FIGO World Congress of Obstetrics and Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:54.
India 2001a {published data only}
-
- Ray A, Mukherjee P, Basu G, Chatterjee A. Misoprostol and third stage of labour. Journal of Obstetrics and Gynecology of India 2001;51(6):53‐4.
India 2005b {published data only}
-
- Singh N, Singh U. Methylergometrine and carboprost tromethamine prophylaxis for postpartum hemorrhage. Journal of Obstetrics and Gynaecology of India 2005;55(4):325‐8.
India 2006e {published data only}
-
- Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. International Journal of Gynecology & Obstetrics 2006;92:170‐5. - PubMed
India 2006h {published data only}
-
- Verma P, Aggarwal N, Jain V, Suri V. A double‐blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S137‐S138. - PubMed
India 2009a {published data only}
-
- Bellad M, Ganachari TDM, Mallapur M. Sublingual (SL) powdered misoprostol (400 mcg) vs IM oxytocin (10 IU) for prevention of postpartum blood loss ‐ a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S124‐S125.
India 2009c {published data only}
-
- Tripti N, Balram S. 400 ug oral misoprostol versus 0.2mg intravenous methyl ergometrine for the active management of third stage of labor. Journal of Obstetrics and Gynecology of India 2009;59(3):228‐34.
Indonesia 2002 {published data only}
-
- Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2002;28(1):46.
Israel 1992 {published data only}
-
- Bider D, Ben‐Rafael Z, Dulitzky M, Menashe Y, Mashiach S, Barkai G. Effect of intraumbilical prostaglandin F2alpha injection on the third stage of labour. Journal of Reproductive Medicine 1992;37:317‐9. - PubMed
Italy 1988 {published data only}
-
- Norchi S, Beretta E, Zanini A, Bottino S. Prevention of primary post‐partum haemorrhage (PPH). Controlled clinical trial: sulprostone vs metilergometrina. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988.
Japan 1976 {published data only}
-
- Takagi S, Yoshida T, Togo Y, Tochigi H, Abe M, Sakata H, et al. The effects of intramyometrial injection of prostaglandin F2alpha on severe post‐partum hemorrhage. Prostaglandins 1976;12:565‐79. - PubMed
Korea 2007 {published data only}
-
- Hong SC, Kim JW, Park HT, Seol HJ, Kim HJ, Kim SH, et al. Additional rectal misoprostol plus intravenous oxytocin versus intravenous oxytocin for the prevention of postpartum hemorrhage after cesarean section. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S99, Abstract no: 321.
Singapore 1990 {published data only}
-
- Ilancheran A, Ratnam SS. Effects of oxytocics on prostaglandin levels in the third stage of labour. Gynecologic and Obstetric Investigation 1990;29:177‐80. - PubMed
Singapore 2001 {published and unpublished data}
-
- Chong YS, Chua S, El‐Refaey H, Choo WL, Boonsri C, Koh BC, et al. Can oral misoprostol be used as an alternative to parenteral oxytocics in the active management of the third stage of labour? A preliminary study of its effect on the postpartum uterus. Personal Communication 1997.
-
- Chong YS, Chua S, El‐Refaey H, Choo WL, Chanrachakul B, Tai BC, et al. Postpartum intrauterine pressure studies of the uterotonic effect of oral misoprostol and intramuscular syntometrine. BJOG: an international journal of obstetrics and gynaecology 2001;108:41‐7. - PubMed
South Africa 1999 {published data only}
-
- Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durham primary postpartum haemorrhage study. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town, South Africa. 1999:77‐8.
-
- Lokugamage AU, Sullivan KR, Niculescu I, Tigere P, Onyangunga F, El‐Refaey H, et al. A randomized study comparing rectally administered misoprostol versus Syntometrine combined with an oxytocin infusion for the cessation of primary post partum hemorrhage. Acta Obstetrica et Gynecologica Scandinavica 2001;80:835‐9. - PubMed
Thailand 2000 {published data only}
-
- Jirakulsawas J, Khooarmompattana S. Comparison of oral misoprostol and intramuscular methylergonovine for prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 2000;12(4):332.
Turkey 2005 {published data only}
-
- Ozkaya O, Sezik M, Kaya H, Desdicioglu R, Dittrich R. Placebo‐controlled randomized comparison of vaginal with rectal misoprostol in the prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2005;31:389‐93. - PubMed
United Kingdom 2001a {published data only}
-
- Acharya G, Al‐Sammarai M, Patel N, Al‐Habib, Kiserud T. A randomised, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra‐operative blood loss during caesarean section. Acta Obstetrcia et Gynecologica Scandinavica 2001;80:245‐50. - PubMed
USA 1983 {published data only}
-
- Nelson GH. Use of 15‐Methyl prostaglandin F2alpha postpartum to contract the uterus in normal pregnant women. Journal of the Medical Association of Georgia 1983;72:703‐6. - PubMed
USA 1999 {published data only}
-
- Daly S, Andolina K, Tolosa JE, Roberts N, Wapner R. A randomized controlled trial of misoprostol versus oxytocin in preventing postpartum blood loss. American Journal of Obstetrics and Gynecology 1999;180:S68.
Yemen 2009 {published data only}
-
- Al‐Harazi AH, Frass KA. Sublingual misoprostol for the prevention of postpartum hemorrhage. Saudi Medical Journal 2009;30(7):912‐6. - PubMed
References to studies awaiting assessment
Afolabi 2010 {published data only}
-
- Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Medical Journal 2010;51(3):207‐11. - PubMed
Carbonell 2009 {published data only}
-
- Carbonell i Esteve JL, Hernandez JMR, Piloto M, Setien SA, Texido CS, Tomasi G, et al. Active management of the third phase of labour plus 400 mug of sublingual misoprostol and 200 mug of rectal misoprostol versus active management only in the prevention of post‐partum haemorrhage. A randomised clinical trial [Manejo activo de la tercera fase del parto mas 400 mug de misoprostol sublingual y 200 mug de misoprostol rectal frente a manejo activo solo en la prevencion de la hemorragia posparto. Ensayo clinico aleatorizado]. Progresos de Obstetricia y Ginecologia 2009;52(10):543‐51.
Chaudhuri 2012 {published data only}
-
- Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low‐risk women. International Journal of Gynecology and Obstetrics 2012;116(2):138‐42. - PubMed
Dong 2011 {published data only}
-
- Dong Y. Effects of carboprost on prevention of hemorrhage after induced labor with scarred uterus. Journal of Shanghai Jiaotong University (Medical Science) 2011;31(8):1212‐5.
Elati 2011 {published data only}
-
- Elati A, Elmahaishi MS, Elmahaishi MO, Elsraiti OA, Weeks AD. The effect of misoprostol on postpartum contractions: a randomised comparison of three sublingual doses. BJOG: an international journal of obstetrics and gynaecology 2011;118(4):466‐73. - PubMed
Kumar 2011 {published data only}
-
- Kumar S. A study to determine the efficacy of 600 mcg misoprostol in prevention of post partum haemorrhage. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:305.
Le 2000 {published data only}
-
- Le J. Prevention of postpartum hemorrhage by carboprost and oxytocin in 90 cases analysis. Acta Medicinae Sinica 2000;13(2):140‐1.
Mansouri 2011 {published data only}
-
- Mansouri HA, Alsahly N. Rectal versus oral misoprostol for active management of third stage of labor: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283(5):935‐9. - PubMed
Owonikoko 2011 {published data only}
-
- Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: A randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(7):715‐21. - PubMed
Sadiq 2011 {published data only}
-
- Sadiq UG, Kwanashie O, Mairiga G, Gamaniel S, Isa H, Abdu A, et al. A randomised clinical trial comparing the efficacy of oxytocin injection and oral misoprostol tablet in the prevention of postpartum haemorrhage in Maiduguri Nigeria. International Research Journal of Pharmacy 2011;2(8):76‐81.
Wang 2000 {published data only}
-
- Wang BI, Du JM. Clinical study on reduction of postpartum bleeding using carprost suppository. Henan Medical Research 2000;9(2):155‐6.
Yan 2000 {published data only}
-
- Yan WG, Ling MX, Mao HY. Clinical study on reduction of postpartum bleeding in cesarean operation by misoprostol. Journal of Zhenjiang Medical College 2000;10(3):440‐1.
Yuan 2003 {published data only}
-
- Yuan YM, Liu CF. Clinical observation of treatment for 60 cases of postpartum hemorrhage with misoprostol tablets combined with jiashenshenghuatang. Chinese Clinical New Medicine 2003;3(10):902.
References to ongoing studies
Kalahroudi 2010 {published data only}
-
- Kalahroudi MA. Comparison of the effect of rectal misoprostol and syntometrin in prevention of post partum hemorrhage. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010).
Moradi 2010 {published data only}
-
- Moradi S. Comparison of misoprostol and oxytocin in reduction of postpartum hemorrhage. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010).
Additional references
Amant 2001
-
- Amant F. The misoprostol third stage study: a randomised controlled comparison between orally administered misoprostol and standard management. [letter]. BJOG: an international journal of obstetrics and gynaecology 2001;108:338. - PubMed
Andolina 1999
-
- Andolina K, Daly S, Roberts N, Tolosa J, Wapner R. Objective measurement of blood loss at delivery: is it more than a guess?. American Journal of Obstetrics and Gynecology 1999;180:S69.
Begley 2011
Carroli 2001
-
- Carroli G. RHL practical aspects: oxytocin or oxytocin+ergot alkaloids in active management of the third stage of labour. The WHO Reproductive Health Library. Oxford: Update Software. WHO/RHR/01.6 2001.
Chong 1997
-
- Chong YS, Chua S, Arulkumaran S. Severe hyperthermia following oral misoprostol in the immediate postpartum period. Obstetrics & Gynecology 1997;90:703. - PubMed
Cotter 2001
Egger 1997
El‐Refaey 1997
-
- El‐Refaey H, O'Brien P, Morafa W, Walder J, Rodeck C. Use of misoprostol in the prevention of postpartum haemorrhage. British Journal of Obstetrics and Gynaecology 1997;104:336‐9. - PubMed
Ghana 2009
-
- Ghana Statistical Service (GSS), Ghana Health Service (GHS), and Macro International. Ghana Maternal Health Survey 2007. Calverton, Maryland, USA: GSS, GHS, and Macro International, 2009.
Gibbens 1972
-
- Gibbens D, Boyd NRH, Crocker S, Baumber S, Chard T. The circulating levels of oxytocin following intravenous and intramuscular administration of syntometrine. Journal of Obstetrics and Gynaecology of the British Commonwealth 1972;79:644‐6. - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. - PubMed
Higgins 2005
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Hofmeyr 2009
Hogerzeil 1996
-
- Hogerzeil HV, Walker GJA. Instability of (methyl)ergometrine in tropical climates: an overview. European Journal of Obsterics & Gynecology and Reproductive Biology 1996;69:25‐9. - PubMed
Khan 2006
-
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PFA. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066‐74. - PubMed
McDonald 2004
PATH 2001
-
- PATH. Oxytocin in pre‐filled UnijectTM injection devices for management of third stage of labour: an introductory trial in Lombok, Indonesia. Final report to Program for Appropriate Technology in Health (PATH), Seattle, Washington, USA, May 2001. PATH, 2001.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Tsu 2009
-
- Tsu VD, Luu HT, Mai TT. Does a novel prefilled injection device make postpartum oxytocin easier to administer? Results from midwives in Vietnam. Midwifery 2009;25(4):461‐5. - PubMed
WHO 1994
-
- Groot ANJA, Vree TB, Hogerzeil HV, Walker GJA. Stability of oral oxytocics in tropical climates (WHO/DAP/94.13). Geneva: World Health Organization, 1994.
WHO 2011
-
- WHO Expert Committee on Essential Medicines. Unedited report of the 18th Expert Committee on the Selection and Use of Essential Medicines. WHO, 2011.
Zieman 1997
-
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD, Zieman M, et al. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. - PubMed
References to other published versions of this review
Gülmezoglu 1997
-
- Gülmezoglu AM. Prostaglandins in third stage of labour. Cochrane Database of Systematic Reviews 1997, Issue 3.
Gülmezoglu 1998
-
- Gülmezoglu AM. Prostaglandins in third stage of labour. Cochrane Database of Systematic Reviews 1998, Issue 4.
Gülmezoglu 2000
-
- Gülmezoglu AM. Prostaglandins for prevention of postpartum haemorrhage. Cochrane Database of Systematic Reviews 2000, Issue 4. - PubMed
Gülmezoglu 2002
Gülmezoglu 2003
-
- Gülmezoglu AM, Forna F, Villar J, Hofmeyr GJ. Prostaglandins for prevention of postpartum haemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000494] - DOI
Gülmezoglu 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

