Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma
- PMID: 22895938
- PMCID: PMC7104534
- DOI: 10.1002/14651858.CD003915.pub4
Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma
Abstract
Background: Although endometrial adenocarcinoma is a common gynaecological cancer, a comparatively small proportion of patients present with, or develop, recurrent or advanced disease. However, for those women whose disease does progress or recur the prognosis is poor and the best treatment is yet to be identified. Co-morbidity, including obesity and cardiac disease, and concerns over toxicity have prevented more extensive studies of cytotoxic chemotherapy, although there are a number of active agents.
Objectives: To assess any benefits or adverse effects of cytotoxic chemotherapy in women with advanced, recurrent or metastatic endometrial adenocarcinoma.
Search methods: Systematic searches of MEDLINE, EMBASE, CENTRAL and the Cochrane Gynaecological Cancer specialist trials register were conducted to identify all eligible randomised controlled trials (RCTs).Databases were searched from 1966 to January 2012. Literature searches were supplemented with searches of relevant trials registers and conference proceedings.
Selection criteria: RCTs comparing chemotherapy versus another intervention (including different chemotherapy) in advanced disease were considered. Trials of adjuvant treatment or for sarcomatous tumours were excluded.
Data collection and analysis: Data were extracted from the papers by review authors and authors of included studies contacted for further information.
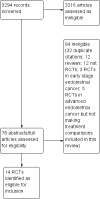
Main results: Fourteen eligible trials, which recruited patients between 1974 and 2005, were identified, eight of which compared 'more' with 'less' chemotherapy. Results from these eight trials, including 1519 patients, showed that treatment consisting of 'more' chemotherapy was associated with longer overall survival (OS) (hazard ratio (HR) 0.86; 95% confidence intervals (CI) 0.77 to 0.96; P = 0.005) and with longer progression-free survival (PFS) (n = 1526; HR 0.82; 95% CI 0.74 to 0.90; P < 0.0001). However, serious acute toxicities were more common in women randomised to the more-intense chemotherapy regimens.There was no evidence to suggest that any particular doublet chemotherapy was better (or worse) than any other, or that any single-agent chemotherapy was better (or worse) than another; however, data for these two comparisons were limited. There were no comparative trials of chemotherapy with endocrine therapy or best supportive care alone.
Authors' conclusions: This review suggests that more-intense chemotherapy regimens may improve both OS and PFS for women with advanced or recurrent endometrial cancer. However, owing to inconsistencies between cytotoxic drug combinations that have been assessed in randomised trials to date, the optimum regimen has still to be defined. Future trials should aim to include measures of quality of life (QoL) and symptom control in addition to survival and progression outcomes.
Conflict of interest statement
None known.
Figures












Update of
-
Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma.Cochrane Database Syst Rev. 2005 Oct 19;(4):CD003915. doi: 10.1002/14651858.CD003915.pub3. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD003915. doi: 10.1002/14651858.CD003915.pub4. PMID: 16235346 Updated.
References
References to studies included in this review
Aapro 2003 {published data only}
-
- Aapro M, Bolis G, Chevallier B, Burg MEL, Poveda A, Oliveira C, et al. An EORTC‐GCCG randomized phase II trial of doxorubicin versus dox‐cisplatin (cDDP) in endometrial carcinoma. Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1994; Vol. 13, issue Abstract 885:275.
-
- Aapro MS, Wijk FH, Bolis G, Chevallier B, Burg MEL, Poveda A, et al on behalf of the EORTC‐GCCG. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC‐GCCG. Annals of Oncology 2003;14:441‐8. - PubMed
Cohen 1984 {published and unpublished data}
-
- Cohen CJ, Bruckner HW, Deppe G, Blessing JA, Homesley H, Lee JH, et al. Multidrug treatment of advanced and recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Obstetrics and Gynecology 1984;63(5):719‐26. - PubMed
Edmonson 1987 {published and unpublished data}
-
- Edmonson JH, Krook JE, Hilton JF, Malkasian GD, Everson LK, Jefferies JA, et al. Randomized phase II studies of cisplatin and a combination of cyclophosphamide‐doxorubicin‐cisplatin (CAP) in patients with progestin‐refractory advanced endometrial carcinoma. Gynecologic Oncology 1987;28(1):20‐4. - PubMed
Fleming 2004a {published data only}
-
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2004;22(11):2159‐66. - PubMed
-
- Fleming GF, Brunetto VL, Mundt AJ, Burks RT, Look KY, Reid G. Randomised trial of doxorubicin (DOX) plus cisplatin (CIS) versus DOX plus CIS plus paclitaxel (TAX) in patients with advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group (GOG) study. Proceedings of American Society of Clinical Oncology. 2002; Vol. 21:202a Abstract 807.
Fleming 2004b {published data only}
-
- Fleming GF, Filiaci VL, Bentley RC, Herzog T, Vaccarello L, Gallion H. Phase III randomised trial of doxorubicin + cisplatin versus doxorubicin + 24‐h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Annals of Oncology 2004;15:1173‐8. - PubMed
Gallion 2003 {published data only}
-
- Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrence endometrial carcinoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2003;21(20):3808‐13. - PubMed
Horton 1978 {published data only}
-
- Horton J, Begg CB, Arseneault J, Bruckner H, Creech R, Hahn RG. Comparison of adriamycin with cyclophosphamide in patients with advanced endometrial cancer. Cancer Treatment Reports 1978;62(1):159‐61. - PubMed
Horton 1982 {published and unpublished data}
-
- Horton J, Elson P, Gordon P, Hahn R, Creech R. Combination chemotherapy for advanced endometrial cancer. An evaluation of three regimens. Cancer 1982;49:2241‐5. - PubMed
Long 2006 {published data only}
-
- Long HJ, Nelimark RA, Cha SS. Comparison of methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) vs doxorubicin and cisplatin (AC) in advanced endometrial carcinoma. Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1995; Vol. 14:282 Abstract 797.
-
- Long III H, Nelimark R, Podratz K, Suman V, Keeney G, Nikcevich D, et al. Phase III comparison of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) vs. doxorubicin and cisplatin (AC) in women with advanced primary or recurrent metastatic carcinoma of the uterine endometrium. Gynecologic Oncology 2006;100:501‐5. - PubMed
Nomura 2010a {published and unpublished data}
-
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: Japanese Gynecologic Oncology Group trial (JGOG2041). Journal of Clinical Oncology (ASCO Meeting Abstracts). 2008; Vol. 26:16526. - PubMed
-
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Annals of Oncology 2011;22(3):636‐42. - PubMed
Nomura 2010b {published and unpublished data}
-
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: Japanese Gynecologic Oncology Group trial (JGOG2041). Journal of Clinical Oncology (ASCO Meeting Abstracts). 2008; Vol. 26:16526. - PubMed
-
- Nomura H, Aoki D, Takahashi F, Katsumata N, Watanabe Y, Konishi I, et al. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041). Annals of Oncology 2011;22(3):636‐42. - PubMed
Pawinski 1999 {published and unpublished data}
-
- Pawinski A, Tumolo S, Hoesel G, Cervantes A, Oosterom AT, Hoctin Boes G, et al. Cyclophosphamide or ifosfamide in patients with advanced and/or recurrent endometrial carcinoma: a randomised phase II study of the EORTC Gynecological Cancer Cooperative Group. European Journal of Obstetrics and Gynecology and Reproductive Biology 1999;86:179‐83. - PubMed
Thigpen 1994 {published data only}
-
- Thigpen J, Blessing P, DiSaia P, Ehrlich C for the Gynecologic Oncology Group. A randomized comparison of adriamycin with or without cyclophosphamide in the treatment of advanced or recurrent endometrial carcinoma. Proceedings of the American Society of Clinical Oncology. 1985:115 Abstract C‐448.
-
- Thigpen JT, Blessing JA, DiSaia PJ, Yordan E, Carson LF, Evers C. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology 1994;12(7):1408‐14. - PubMed
Thigpen 2004 {published data only}
-
- Thigpen JT, Brady MF, Homesley, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecologic Oncology Group Study (GOG). Journal of Clinical Oncology 2004;22(19):3902‐8. - PubMed
-
- Thigpen T, Blessing J, Homesley H, Malfetano J, DiSaia P, Yordan E. Phase III trial of doxorubicin ± cisplatin in advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group (GOG) study. Proceedings of the American Society of Clinical Oncology. 1993; Vol. 12:261 Abstract 830.
Weber 2003 {published data only}
-
- Weber B, Mayer F, Bougnoux P, Lesimple T, Joly F, Fabbro M, et al. What is the best chemotherapy regimen in recurrent or advanced endometrial carcinoma? Preliminary results. Proceedings of the American Society of Clinical Oncology. 2003; Vol. 22:Abstract 1819.
References to ongoing studies
NCT00052312 {unpublished data only}
-
- Reed NS (Investigator). Doxorubicin and cisplatin with or without paclitaxel in treating patients with locally advanced, metastatic and/or relapsed endometrial cancer. http://clinicaltrials.gov/ct2/show/record/NCT00052312.
NCT00063999 {unpublished data only}
-
- Miller D (Protocol chair). Randomized phase III trial of doxorubicin/cisplatin/paclitaxel and G‐CSF versus carboplatin/paclitaxel in patients with stage III and IV or recurrent endometrial cancer. http://clinicaltrials.gov/ct/show/NCT0006399.
Additional references
Bremond 2001
Burke 1990
-
- Burke TW, Heller PB, Woodward J, Davidson SA, Hoskins WJ, Park RC, et al. Treatment failure in endometrial carcinoma. Obstetrics and Gynaecology 1990;75:96‐101. - PubMed
Cullen 1999
-
- Cullen MH, Woodroffe CM, Billingham LJ, Chetiyarwardana AD, Joshi R, Ferry D, et al. Mitomycin, ifosfamide and cisplatin (MIC) in unresectable non‐small cell lung cancer: effects on survival and quality of life. Journal of Clinical Oncology 1999;17(10):3188‐94. - PubMed
EBCTCG 1998
-
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. The Lancet 1998;351:1451‐67. - PubMed
Faust 2010
-
- Faust G, Davies Q, Symonds P. Changes in the treatment of endometrial cancer. British Journal of Obstetrics and Gynaecology 2010;117:1043‐6. - PubMed
FIGO 2009
-
- Creasman W. Revised FIGO staging for carcinoma of the endometrium. International Journal of Gynaecology and Obstetrics 2009;105(2):109. - PubMed
Jemal 2011
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA: Cancer Journal for Clinicians 2011;61:69‐90. - PubMed
Johnson 2011
Kokka 2010
Miller 1981
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. - PubMed
Morris 1996
-
- Morris M, Alvarez RD, Kinney WK, Wilson TO. Treatment of recurrent adenocarcinoma of the endometrium with pelvic exenteration. Gynecologic Oncology 1996;60:288. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Tierney 2007
Yusuf 1985
-
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. - PubMed
References to other published versions of this review
Humber 2005
Humber 2007
-
- Humber CE, Tierney JF, Symonds RP, Collingwood M, Kirwan J, Williams C, et al. Chemotherapy for advanced, recurrent or metastatic endometrial cancer: a systematic review of Cochrane collaboration. Annals of Oncology 2007;18(3):409‐20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

