Vaccines for preventing influenza in healthy children
- PMID: 22895945
- PMCID: PMC6478137
- DOI: 10.1002/14651858.CD004879.pub4
Vaccines for preventing influenza in healthy children
Update in
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
Abstract
Background: The consequences of influenza in children and adults are mainly absenteeism from school and work. However, the risk of complications is greatest in children and people over 65 years of age.
Objectives: To appraise all comparative studies evaluating the effects of influenza vaccines in healthy children, assess vaccine efficacy (prevention of confirmed influenza) and effectiveness (prevention of influenza-like illness (ILI)) and document adverse events associated with influenza vaccines.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3) which includes the Acute Respiratory Infections Group's Specialised Register, OLD MEDLINE (1950 to 1965), MEDLINE (1966 to November 2011), EMBASE (1974 to November 2011), Biological Abstracts (1969 to September 2007), and Science Citation Index (1974 to September 2007).
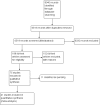
Selection criteria: Randomised controlled trials (RCTs), cohort and case-control studies of any influenza vaccine in healthy children under 16 years of age.
Data collection and analysis: Four review authors independently assessed trial quality and extracted data.
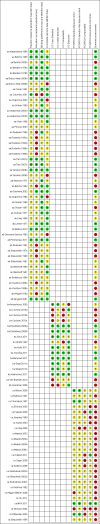
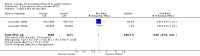
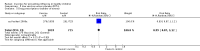
Main results: We included 75 studies with about 300,000 observations. We included 17 RCTs, 19 cohort studies and 11 case-control studies in the analysis of vaccine efficacy and effectiveness. Evidence from RCTs shows that six children under the age of six need to be vaccinated with live attenuated vaccine to prevent one case of influenza (infection and symptoms). We could find no usable data for those aged two years or younger.Inactivated vaccines in children aged two years or younger are not significantly more efficacious than placebo. Twenty-eight children over the age of six need to be vaccinated to prevent one case of influenza (infection and symptoms). Eight need to be vaccinated to prevent one case of influenza-like-illness (ILI). We could find no evidence of effect on secondary cases, lower respiratory tract disease, drug prescriptions, otitis media and its consequences and socioeconomic impact. We found weak single-study evidence of effect on school absenteeism by children and caring parents from work. Variability in study design and presentation of data was such that a meta-analysis of safety outcome data was not feasible. Extensive evidence of reporting bias of safety outcomes from trials of live attenuated influenza vaccines (LAIVs) impeded meaningful analysis. One specific brand of monovalent pandemic vaccine is associated with cataplexy and narcolepsy in children and there is sparse evidence of serious harms (such as febrile convulsions) in specific situations.
Authors' conclusions: Influenza vaccines are efficacious in preventing cases of influenza in children older than two years of age, but little evidence is available for children younger than two years of age. There was a difference between vaccine efficacy and effectiveness, partly due to differing datasets, settings and viral circulation patterns. No safety comparisons could be carried out, emphasising the need for standardisation of methods and presentation of vaccine safety data in future studies. In specific cases, influenza vaccines were associated with serious harms such as narcolepsy and febrile convulsions. It was surprising to find only one study of inactivated vaccine in children under two years, given current recommendations to vaccinate healthy children from six months of age in the USA, Canada, parts of Europe and Australia. If immunisation in children is to be recommended as a public health policy, large-scale studies assessing important outcomes, and directly comparing vaccine types are urgently required. The degree of scrutiny needed to identify all global cases of potential harms is beyond the resources of this review. This review includes trials funded by industry. An earlier systematic review of 274 influenza vaccine studies published up to 2007 found industry-funded studies were published in more prestigious journals and cited more than other studies independently from methodological quality and size. Studies funded from public sources were significantly less likely to report conclusions favourable to the vaccines. The review showed that reliable evidence on influenza vaccines is thin but there is evidence of widespread manipulation of conclusions and spurious notoriety of the studies. The content and conclusions of this review should be interpreted in the light of this finding.
Conflict of interest statement
Tom Jefferson was an ad hoc consultant for F. Hoffman‐La Roche Ltd in 1998 to 1999 on oseltamivir. He receives royalties from his books published by Blackwell and Il Pensiero Scientifico Editore, none of which are on neuraminidase inhibitors. He is occasionally interviewed by market research companies for anonymous interviews about Phase 1 or 2 products unrelated to influenza. He is acting as expert witness for the plaintiff in a legal case for claimed damages after exposure to a pandemic monovalent vaccine.
Figures












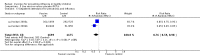











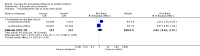



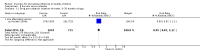
Update of
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2008 Apr 16;(2):CD004879. doi: 10.1002/14651858.CD004879.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD004879. doi: 10.1002/14651858.CD004879.pub4. PMID: 18425905 Updated.
References
References to studies included in this review
-
- Alexandrova GI, Budilovsky GN, Koval TA, Polezhaev FI, Garmashova LM, Ghendon YuZ, et al. Study of live recombinant cold‐adapted influenza bivalent vaccine of type A for use in children: an epidemiological control trial. Vaccine 1986;4(2):114‐8. - PubMed
-
- Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, et al. The efficacy of live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine in children. New England Journal of Medicine 1998;338(20):1405‐12. - PubMed
- Piedra PA, Yan L, Kotloff K, Zangwill K, Bernstein DI, King J, et al. Safety of the trivalent, cold‐adapted influenza vaccine in preschool‐aged children. Pediatrics 2002;110(4):662‐72. - PubMed
-
- Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, et al. Efficacy of vaccination with live attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. Journal of Paediatrics 2000;136(2):168‐75. - PubMed
- Piedra PA, Yan L, Kotloff K, Zangwill K, Bernstein DI, King J, et al. Safety of the trivalent, cold‐adapted influenza vaccine in preschool‐aged children. Pediatrics 2002;110(4):662‐72. - PubMed
-
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase‐specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. Journal of Infectious Diseases 1979;140(6):844‐50. - PubMed
-
- Beutner KR, Chow T, Rubi E, Strussenberg J, Clement J, Ogra PL. Evaluation of a neuraminidase‐specific influenza A virus vaccine in children: antibody responses and effects on two successive outbreaks of natural infection. Journal of Infectious Diseases 1979;140(6):844‐50. - PubMed
References to studies excluded from this review
-
- Anonymous. FluMist: an intranasal live influenza vaccine. Medical Letter on Drugs and Therapeutics 2003;45(1163):65‐6. - PubMed
-
- Beare AS, Hobson D, Reed SE, Tyrrell DA. A comparison of live and killed influenza‐virus vaccines. Report to the Medical Research Council's Committee on Influenza and other Respiratory Virus Vaccines. Lancet 1968;2(7565):418‐22. - PubMed
-
- Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, Reisinger K, et al. Correlates of immune protection induced by live, attenuated, cold‐adapted, trivalent, intranasal influenza virus vaccine. Journal of Infectious Diseases 2000;181(3):1133‐7. - PubMed
Additional references
-
- American Academy of Pediatrics Committee on Infectious Diseases. Recommendations for influenza immunization of children. Pediatrics 2004;113:1441‐7. - PubMed
-
- Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta‐analysis of 8 randomized controlled studies. Vaccine 2012;30(5):886‐92. - PubMed
-
- Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011;71(12):1591‐622. - PubMed
-
- Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. Morbidity and Mortality Weekly Report 2007;56(RR‐6):1‐54. - PubMed
References to other published versions of this review
-
- Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet 2005;365(9461):773‐80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

