Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer
- PMID: 22895947
- PMCID: PMC4050358
- DOI: 10.1002/14651858.CD005343.pub3
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer
Update in
-
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2019 Oct 31;2019(10):CD005343. doi: 10.1002/14651858.CD005343.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Feb 5;2:CD005343. doi: 10.1002/14651858.CD005343.pub5. PMID: 31684686 Free PMC article. Updated.
Abstract
Background: Epithelial ovarian cancer presents at an advanced stage in the majority of women. These women require surgery and chemotherapy for optimal treatment. Conventional treatment is to perform surgery first and then give chemotherapy. However, it is not yet clear whether there are any advantages to using chemotherapy before surgery.
Objectives: To assess whether there is an advantage to treating women with advanced epithelial ovarian cancer with chemotherapy before cytoreductive surgery (neoadjuvant chemotherapy (NACT)) compared with conventional treatment where chemotherapy follows maximal cytoreductive surgery.
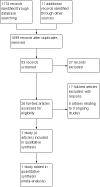
Search methods: For the original review we searched, the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3, 2006), MEDLINE (Silver Platter, from 1966 to 1 Sept 2006), EMBASE via Ovid (from 1980 to 1 Sept 2006), CANCERLIT (from 1966 to 1 Sept 2006), PDQ (search for open and closed trials) and MetaRegister (most current search Sept 2006). For this update randomised controlled trials (RCTs) were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3, 2011) and the Cochrane Gynaecological Cancer Specialised Register (2011), MEDLINE (August week 1, 2011), EMBASE (to week 31, 2011), PDQ (search for open and closed trials) and MetaRegister (August 2011).
Selection criteria: RCTs of women with advanced epithelial ovarian cancer (Federation of International Gynaecologists and Obstetricians (FIGO) stage III/IV) who were randomly allocated to treatment groups that compared platinum-based chemotherapy before cytoreductive surgery with platinum-based chemotherapy following cytoreductive surgery.
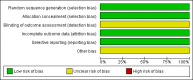
Data collection and analysis: Data were extracted by two review authors independently, and the quality of included trials was assessed by two review authors independently.
Main results: One high-quality RCT met the inclusion criteria. This multicentre trial randomised 718 women with stage IIIc/IV ovarian cancer to NACT followed by interval debulking surgery (IDS) or primary debulking surgery (PDS) followed by chemotherapy. There were no significant differences between the study groups with regard to overall survival (OS) (670 women; HR 0.98; 95% CI 0.82 to 1.18) or progression-free survival (PFS) (670 women; HR 1.01; 95% CI 0.86 to 1.17).Significant differences occurred between the NACT and PDS groups with regard to some surgically related serious adverse effects (SAE grade 3/4) including haemorrhage (12 in NACT group vs 23 in PDS group; RR 0.50; 95% CI 0.25 to 0.99), venous thromboembolism (none in NACT group vs eight in PDS group; RR 0.06; 95% CI 0 to 0.98) and infection (five in NACT group vs 25 in PDS group; RR 0.19; 95% CI 0.07 to 0.50). Quality of life (QoL) was reported to be similar for the NACT and PDS groups.Three ongoing RCTs were also identified.
Authors' conclusions: We consider the use of NACT in women with stage IIIc/IV ovarian cancer to be a reasonable alternative to PDS, particularly in bulky disease. With regard to selecting who will benefit from NACT, treatment should be tailored to the patient and should take into account resectability, age, histology, stage and performance status. These results cannot be generalised to women with stage IIIa and IIIb ovarian cancer; in these women, PDS is the standard. We await the results of three ongoing trials, which may change these conclusions.
Conflict of interest statement
Sean Kehoe is lead investigator in the CHORUS # study.
Figures







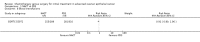
Update of
-
Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD005343. doi: 10.1002/14651858.CD005343.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD005343. doi: 10.1002/14651858.CD005343.pub3. PMID: 17943850 Updated.
References
References to studies included in this review
-
- Vergote I, Amant F, Kristensen G, Ehlen T, Reed NS, Casado A. Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. European Journal of Cancer 2011;47(Suppl 3):S88‐91. - PubMed
- Vergote I, Pecorelli S, Stuart G. Intergroup Study (EORTC 55971/NCIC OV13). Randomized Phase III study comparing upfront debulking surgery versus neo‐adjuvant chemotherapy in patients with Stage IIIc or IV epithelial ovarian carcinoma. Trial protocol: http://www.cancer.gov/clinicaltrials/EORTC‐55971 2003 (accessed 17 June 2012).
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine 2010;363(10):943‐53. [Incl. Supplementary Appendix and Protocol] - PubMed
- Vergote I, Tropé CG, Amant F, Ehlen T, Reed NS, Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. Journal of Clinical Oncology 2011;29(31):4076‐8. - PubMed
- Verleye L, Ottevanger PB, Kristensen GB, Ehlen T, Johnson N, Burg ME, et al. Quality of pathology reports for advanced ovarian cancer: are we missing essential information? An audit of 479 pathology reports from the EORTC‐GCG 55971/NCIC‐CTG OV13 neoadjuvant trial. European Journal of Cancer 2011;47(1):57‐64. - PubMed
References to studies excluded from this review
-
- Ansquer Y, Leblanc E, Clough K, Morice P, Dauplat J, Mathevet P, et al. Neoadjuvant chemotherapy for unresectable ovarian carcinoma: a French multicenter study. Cancer 2001;91(12):2329‐34. - PubMed
-
- Baekelandt M. The potential role of neoadjuvant chemotherapy in advanced ovarian cancer. International Journal of Gynecological Cancer 2003;13 Suppl 2:163‐8. - PubMed
-
- Bertelsen K. Tumor reduction surgery and long‐term survival in advanced ovarian cancer: a DACOVA study. Gynecologic Oncology 1990;38(2):203‐9. - PubMed
-
- Bidzinski M, Danska‐Bidzinska A, Ziólkowska‐Seta I, Derlatka P, Sobiczewski P, Raczynski P. Analysis of the treatment of ovarian cancer patients with neo‐adjuvant chemotherapy ‐ preliminary results. European Journal of Gynaecological Oncology 2005;26(4):423‐6. - PubMed
-
- Bristow R, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival impact of maximum cytoreductive surgery for advanced ovarian carcinoma during the platinum‐era: a meta‐analysis of 6,848 patients. Proceedings of the American Society of Clinical Oncology. 2001; Vol. 20:(Abstract 807) 202a.
References to ongoing studies
-
- Kehoe S, Wheeler S. CHORUS (Chemotherapy or Upfront Surgery). A randomised feasibility trial to determine the impact of timing of surgery and chemotherapy in newly diagnosed patients with advanced epithelial ovarian, primary peritoneal or fallopian tube carcinoma. www.ctu.mrc.ac.uk/plugins/StudyDisplay/protocols/CHORUS protocol Version.... London, (accessed 18/6/2012).
- Law K, Murray C, Kehoe S. CHORUS ‐ a randomised study to determine the impact of timing of surgery and chemotherapy in newly diagnosed patients with advanced epithelial ovarian, primary peritoneal or fallopian tube carcinoma. Proceedings of the Annual Meeting of the British Gynaecological Cancer S...90.
-
- Janga D, Kumar L, Kumar S, Shukla NK, Thulkar S, Singh R. Neoadjuvant chemotherapy (CT) followed by debulking surgery vs upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma (EOC): a prospective, randomized study. Proceedings of the American Society of Clinical Oncology. 2003; Vol. 22:487.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Shukla N, Thulkar S, et al. Neoadjuvant chemotherapy in advanced epithelial ovarian cancer (EOC): a phase III randomized study. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I (June 20 Supplement). 2006; Vol. 24:18 Suppl.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Shukla NJ. Neo‐adjuvant chemotherapy in advanced epithelial ovarian cancer (EOC): a prospective, randomized study. Indian Journal of Medical and Paediatric Oncology 2009;30(1):15.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Vijayaraghavan M. Neoadjuvant chemotherapy followed by interval debulking surgery versus upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma: a prospective randomized study ‐ interim results. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings Part I. Chicago, Illinois, 2007; Vol. 25(18 Suppl):5531.
- Kumar L, Hariprasad R, Kumar S, Bhatla N, Thulkar S, Vijayaraghavan M, et al. Neoadjuvant chemotherapy followed by interval debulking surgery versus upfront surgery followed by chemotherapy in advanced epithelial ovarian carcinoma: a prospective randomized study ‐ interim results. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement). 2007:5531.
- Kumar L, Janga D, Berge S, Gupta S, Kumar S, Bhatla N, et al. Neoadjuvant chemotherapy in stage III & IV epithelial ovarian carcinoma (EOC). Journal International Medical Sciences Academy 2003;16(2):89‐92.
-
- Onda T, Matsumoto K, Shibata T, Sato A, Fukuda H, Konishi I, et al. Phase III trial of upfront debulking surgery versus neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers: Japan Clinical Oncology Group Study JCOG0602. Japanese Journal of Clinical Oncology 2008;38(1):74‐7. - PubMed
Additional references
-
- Allen DG, Heintz AP, Touw FW. A meta‐analysis of residual disease and survival in stage III and IV carcinoma of the ovary. European Journal of Gynaecologic Oncology 1995;16(5):349‐56. - PubMed
-
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta‐analysis. Journal of Clinical Oncology 2002;20(5):1248‐59. - PubMed
-
- Bristow RE, Chi DS. Platinum‐based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta‐analysis. Gynecologic Oncology 2006;103(3):1070‐6. - PubMed
-
- Bristow RE, Eisenhauer EL, Santillan A, Chi DS. Delaying the primary surgical effort for advanced ovarian cancer: a systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecologic Oncology 2007;104:480‐90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

