Nicotine vaccines for smoking cessation
- PMID: 22895958
- PMCID: PMC6486305
- DOI: 10.1002/14651858.CD007072.pub2
Nicotine vaccines for smoking cessation
Abstract
Background: By reducing the amount of nicotine that reaches the brain when a person smokes a cigarette, nicotine vaccines may help people to stop smoking or to prevent recent quitters from relapsing.
Objectives: The aims of this review are to assess the efficacy of nicotine vaccines for smoking cessation and for relapse prevention, and to assess the frequency and type of adverse events associated with the use of nicotine vaccines.
Search methods: We searched the Cochrane Tobacco Addiction Review Group specialised register for trials, using the term 'vaccine' in the title or abstract, or in a keyword (date of most recent search April 2012). To identify any other material including reviews and papers potentially relevant to the background or discussion sections, we also searched MEDLINE, EMBASE, and PsycINFO, combining terms for nicotine vaccines with terms for smoking and tobacco use, without design limits or limits for human subjects. We searched the Annual Meeting abstracts of the Society for Research on Nicotine and Tobacco up to 2012, using the search string 'vaccin'. We searched Google Scholar for 'nicotine vaccine'. We also searched company websites and Google for information related to specific vaccines. We searched clinicaltrials.gov in March 2012 for 'nicotine vaccine' and for the trade names of known vaccine candidates.
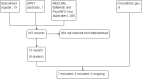
Selection criteria: We included randomized controlled trials of nicotine vaccines, at Phase II and Phase III trial stage and beyond, in adult smokers or recent ex-smokers. We included studies of nicotine vaccines used as part of smoking cessation or relapse prevention interventions.
Data collection and analysis: We extracted data on the type of participants, the dose and duration of treatment, the outcome measures, the randomization procedure, concealment of allocation, blinding of participants and personnel, reporting of outcomes, and completeness of follow-up.Our primary outcome measure was a minimum of six months abstinence from smoking. We used the most rigorous definition of abstinence, and preferred cessation rates at 12 months and biochemically validated rates where available. We have used the risk ratio (RR) to summarize individual trial outcomes. We have not pooled the current group of included studies as they cover different vaccines and variable regimens.
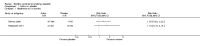
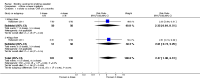
Main results: There are no nicotine vaccines currently licensed for public use, but there are a number in development. We found four trials which met our inclusion criteria, three comparing NicVAX to placebo and one comparing NIC002 (formerly NicQbeta) to placebo. All were smoking cessation trials conducted by pharmaceutical companies as part of the drug development process, and all trials were judged to be at high or unclear risk of bias in at least one domain. Overall, 2642 smokers participated in the included studies in this review. None of the four included studies detected a statistically significant difference in long-term cessation between participants receiving vaccine and those receiving placebo. The RR for 12 month cessation in active and placebo groups was 1.35 (95% Confidence Interval (CI) 0.82 to 2.22) in the trial of NIC002 and 1.74 (95% CI 0.73 to 4.18) in one NicVAX trial. Two Phase III NicVAX trials, for which full results were not available, reported similar quit rates of approximately 11% in both groups. In the two studies with full results available, post hoc analyses detected higher cessation rates in participants with higher levels of nicotine antibodies, but these findings are not readily generalisable. The two studies with full results showed nicotine vaccines to be well tolerated, with the majority of adverse events classified as mild or moderate. In the study of NIC002, participants receiving the vaccine were more likely to report mild to moderate adverse events, most commonly flu-like symptoms, whereas in the study of NicVAX there was no significant difference between the two arms. Information on adverse events was not available for the large Phase III trials of NicVAX.Vaccine candidates are likely to undergo significant changes before becoming available to the general public, and those included in this review may not be the first to reach market; this limits the external validity of the results reported in this review in terms of both effectiveness and tolerability.
Authors' conclusions: There is currently no evidence that nicotine vaccines enhance long-term smoking cessation. Rates of serious adverse events recorded in the two trials with full data available were low, and the majority of adverse events reported were at mild to moderate levels. The evidence available suggests nicotine vaccines do not induce compensatory smoking or affect withdrawal symptoms. No nicotine vaccines are currently licensed for use in any country but a number are under development.Further trials of nicotine vaccines are needed, comparing vaccines with placebo for smoking cessation. Further trials are also needed to explore the potential of nicotine vaccines to prevent relapse. Results from past, current and future research should be reported in full. Adverse events and serious adverse events should continue to be carefully monitored and thoroughly reported.
Conflict of interest statement
Jacques Cornuz and Dorothy Hatsukami are principal investigators of trials included in this review.
Dorothy Hatsukami has received funding from Nabi Biopharmaceuticals to conduct clinical Phase II and III trials testing the nicotine vaccine, NicVAX. She also attended a meeting convened by Selecta Biosciences to discuss a nicotine vaccine that is being developed by this company. No honoraria have been received from either of these companies although costs for travel have been covered.
Jacques Cornuz has received funding from Cytos to conduct a clinical Phase II trial testing the nicotine vaccine, NicQb [NIC002]. He attended two international scientific meetings to present the results of such a trial developed by this company. Costs of travel have been covered by Cytos.
Jamie Hartmann‐Boyce and Kate Cahill have no conflicts of interest to declare for this review.
Figures





Update of
References
References to studies included in this review
Cornuz 2008 {published data only}
-
- Cornuz J, Klingler K, Mueller P, Jungi F, Cerny T. A therapeutic vaccine for nicotine dependence: results of a phase I and a randomized phase II study [abstract]. Annual Meeting Proceedings of the American Society of Clinical Oncology 2005;23:108.
-
- Cytos Biotechnology. NIC002: a novel vaccine for nicotine addiction. http://www.cytos.ch/doc/NIC002_Nicaddiction_Facts_August07.pdf (accessed 25 Feb /2012) 2007.
-
- Willers J. Clinical trial protocol synopsis: Evaluation of efficacy and safety of Nicotine‐Qbeta (NicQb) vaccine versus placebo in healthy smokers. http://www.plosone.org/article/info:doi%2F10.1371%2Fjournal.pone.0002547... (accessed 15 March 2012) 2004.
Hatsukami 2011 {published data only}
-
- Fahim R. Promising new treatments including vaccines. http://www.seiservices.com/blendingalbuquerque/doc/R.Fahim%20Session%201... 2010.
-
- Rennard SI, Jorenby JE, Gonzales D, Rigotti NA, Vos A, Bortey EB. Abstract 3712: A randomized placebo‐controlled trial of a conjugate nicotine vaccine (Nic‐VAX®) in smokers who want to quit: 12 month results. Circulation 2007;116:ii_844.
-
- Rigotti N, Reus V, Glover E, Tashkin D, Oncken C, Rennard S, et al. Nicotine vaccine vs placebo for smoking cessation: long‐term abstinence and quitting [POS5‐1]. Society for Research on Nicotine and Tobacco 14th Annual Meeting February 27‐ March 1, Portland, Oregon. Rapid Communication Abstracts. 2008.
NCT00836199 {published and unpublished data}
-
- Nabi Biopharmaceuticals. Clinical Trials: NicVAX® (Nicotine Conjugate Vaccine). http://www.nabi.com/pipeline/clinicaltrials.php (accessed 15 March 2012).
-
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of First NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1586001&... (accessed 15 March 2012) 2011.
NCT01102114 {published and unpublished data}
-
- Nabi Biopharmaceuticals. Clinical Trials: NicVAX® (Nicotine Conjugate Vaccine). http://www.nabi.com/pipeline/clinicaltrials.php (accessed 15 March 2012).
-
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of Second NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1626882&... (accessed 15 March 2012) 2011.
References to studies excluded from this review
Bunce 2005 {published data only}
-
- Bunce C, Ackrel S, Fryer S, Rameal S, Treasure P, Long S. Interim data from a phase i study to identify the optimal dose of the nicotine vaccine, TA‐NIC to use for efficacy trials to establish impact of vaccine on quit rates [RP‐073]. Society for Research on Nicotine and Tobacco 11th annual meeting, March 2005; Prague, Czech Republic. Rapid Communications posters. 2005:19.
-
- Xenova Group PLC. News release: Anti‐smoking vaccine TA‐NIC preliminary 12 month clinical trial results. http://hugin.info/133161/R/982993/146255.pdf (accessed 15 March 2012) 2005.
Hatsukami 2005a {published data only}
-
- Hatsukami D, Rennard S, Jorenby D, Pentel P, Vos A, Horwith G. Results of a phase 2, multi‐center, randomized, double‐blinded, placebo‐controlled, parallel‐arm, dose comparison study to assess the immunogenicity and safety of 3'‐aminomethylnicotine‐P.aeruginosa R‐exoprotein A conjugate vaccine (Nicvax) administered to smokers. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
-
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology and Therapeutics 2005;78(5):456‐67. - PubMed
Lindmayer 2003 {unpublished data only}
-
- Lindamyer K, Horwith G, Fattom A, Naso R, Fuller S, Muenz L, et al. Results of a phase I, double‐blinded, controlled safety and immunogenicity trial of Nicvax, a conjugated nicotine vaccine [POS1‐30]. Society for Research on Nicotine and Tobacco 9th Annual Meeting, New Orleans LA. Feb 19‐22 2003.
Maurer 2005 {published data only}
-
- Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, et al. A therapeutic vaccine for nicotine dependence: pre‐clinical efficacy, and phase I safety and immunogenicity. European Journal of Immunology 2005;35:2031‐40. - PubMed
Nabi 2008 {published data only}
-
- Nabi Biopharmaceuticals. Clinical Trials: NicVAX® (Nicotine Conjugate Vaccine). http://www.nabi.com/pipeline/clinicaltrials.php (accessed 15 March 2012).
St Clair Roberts 2003 {unpublished data only}
-
- Clair Roberts J, Akers CVR, Vanhinsbergh L, McKenna KA, Wood DM, Jack LM. Longitudinal safety and immunogenicity data of Ta‐Nic, a novel nicotine vaccine [POS1‐30]. Society for Research on Nicotine and Tobacco 9th Annual Meeting, New Orleans LA. Feb 19‐22 2003.
Wagena 2008 {published data only}
-
- Wagena EJ, Vos A, Akhavain R, Winkens R, Schayck CP. The safety and immunogenicity of a conjugated nicotine vaccine (Nicvax[TM]): Preliminary results of a randomised placebo‐controlled trial in healthy smokers and non‐smokers. Society for Research on Nicotine and Tobacco 5th European Meeting November 20‐22 2003 Padua: Abstract book. 2003.
-
- Wagena EJ, Vos A, Horwith G, Schayck CP. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo‐controlled 1/2 trial. Nicotine & Tobacco Research 2008;10(1):213‐8. - PubMed
-
- Wagena EJ, Schayck CP, Akhavasin R, Vos A. The safety of immunogenicity of a conjugated nicotine vaccine results from a double blind placebo controlled trial in healthy smokers and non smokers [Abstract]. European Respiratory Journal 2004;24(Suppl 48):28s.
-
- Wagena EJ, Schayck O, Akhavain R, Vos A. Preliminary results of a phase 2 safety trial with nicvax, a conjugated nicotine vaccine. A double‐blind, placebo‐controlled study in healthy smokers and non‐smokers (POS2‐068). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004:79.
References to ongoing studies
NCT00633321 {unpublished data only}
-
- Vaccine: TA‐NICStudy of TA‐NIC to Assess the Efficacy and Safety of the Vaccine as an Aid to Smoking Cessation. Ongoing study May 2007.
NCT00736047 {published and unpublished data}
-
- Cytos Biotechnology. Media Release: Interim analysis of an ongoing phase II study with nicotine vaccine shows primary endpoint not achieved. http://www.cytos.com/userfiles/file/Cytos_Press_E_091015.pdf (accessed 15 March 2012) 2009.
NCT00995033 {unpublished data only}
-
- Vaccine: NicVAXEfficacy and Safety of NicVAX® Co‐administered With Varenicline (Champix®). Ongoing study October 2009.
NCT01178346 {unpublished data only}
-
- Vaccine: NicVAXHealth‐Related Quality‐of‐Life (HRQoL) and Health‐Care Resource Utilization Assessment in Nabi‐4514 and Nabi‐4515 Phase 3 Studies. Ongoing study July 2010.
NCT01280968 {unpublished data only}
-
- Vaccine: NIC002Improving the Efficacy of Anti‐Nicotine Immunotherapy. Ongoing study December 2010.
NCT01304810 {unpublished data only}
-
- Vaccine: NicVAXA Follow‐up Study to Assess Long‐term Immunogenicity and Safety of Nic‐Vax/Placebo as an Aid to Smoking Cessation. Ongoing study January 2011.
Additional references
Balfour 2004
-
- Balfour DJK. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus. Nicotine & Tobacco Research 2004;6(6):899‐912. - PubMed
Benowitz 2010
Bloomberg 2012
-
- Bloomberg Businessweek. Company Overview of Independent Pharmaceutica AB. http://investing.businessweek.com/research/stocks/private/snapshot.asp?p... (accessed 29 March 2012) 2012.
Brozek 2004
-
- Brozek A. Grant given for research on possible nicotine vaccine. Daily Nebraskan News 2004:http://www.dailynebraskan.com/news/grant‐given‐for‐research‐on‐possible‐... (accessed 29 March 2012).
Buckley 2011
-
- Buckley C. A Vaccine for Nicotine?. UConn Today 3 October 2011:http://today.uconn.edu/blog/2011/10/a‐vaccine‐for‐nicotine/ (accessed 29 March 2012).
Cahill 2012
Caponnetto 2012
Cerny 2009
-
- Cerny EH, Cerny T. Vaccines against nicotine. Human Vaccines 2009;5(4):200‐5. - PubMed
Cytos 2007
-
- Cytos Biotechnology. NIC002: a novel vaccine for nicotine addiction. http://www.cytos.ch/doc/NIC002_Nicaddiction_Facts_August07.pdf (accessed 25 Feb 2012) 2007.
Cytos 2009
-
- Cytos Biotechnology. Media Release: Interim analysis of an ongoing phase II study with nicotine vaccine shows primary endpoint not achieved. http://www.cytos.com/userfiles/file/Cytos_Press_E_091015.pdf (accessed 15 March 2012) 2009.
Fagerström 2005
-
- Fagerström K, Balfour DJK. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opinion on Investigational Drugs 2005;15(2):107‐16. - PubMed
Fahim 2011
-
- Fahim RE, Kessler PD, Fuller SA, Kalnik MW. Nicotine vaccines. CNS & Neurological Disorders Drugs Targets 2011;10(8):905‐15. - PubMed
GlaxoSmithKline 2009
-
- GSK and Nabi announce agreement for NicVAX®, a vaccine for nicotine addiction. Press Release Archive 16 November 2009:http://www.gsk.com/media/pressreleases/2009/2009_pressrelease_10130.htm (accessed 15 March 2012).
Hall 2011
-
- Hall W, Gartner C. Ethical and policy issues in using vaccines to treat and prevent cocaine and nicotine dependence. Current Opinion in Psychiatry 2011;24(3):191‐6. - PubMed
Haney 2004
-
- Haney M, Kosten TR. Therapeutic vaccines for substance abuse. Expert Review of Vaccines 2004;3:11‐18. - PubMed
Harvey 2004
-
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug‐taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175:134‐42. - PubMed
Hasman 2004
Hatsukami 2005b
-
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology and Therapeutics 2005;78(5):456‐67. - PubMed
Hicks 2012
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 12 March 2012).
Hughes 2007
Keyler 1999
-
- Keyler DE, Hieda Y, Peter J, Pentel PR. Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine & Tobacco Research 1999;1(3):241‐9. - PubMed
Lancaster 2005
Laviolette 2004
-
- Laviolette SR, Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nature Reviews: Neuroscience 2004;5:55‐65. - PubMed
LeSage 2006
Long 2011
-
- Long M. Quest for Vaccines to Treat Addiction. Wall Street Journal 3 May 2011:http://online.wsj.com/article/SB1000142405274870443600457629898073946339... (accessed 15 March 2012).
Nabi 2011a
-
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of First NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1586001&... (accessed 15 March 2012) 2011.
Nabi 2011b
-
- Nabi Biopharmaceuticals. News Release: Nabi Biopharmaceuticals Announces Results of Second NicVAX(R) Phase III Clinical Trial. http://phx.corporate‐ir.net/phoenix.zhtml?c=100445&p=irol‐newsArticle&ID=1626882&... (accessed 15 March 2012) 2011.
Nabi 2012
-
- Q4 2011 Nabi Biopharmaceuticals Earnings Conference Call. http://edge.media‐server.com/m/p/sw2pg4dk/lan/en (accessed 15 March 2012) 14 March 2012.
NIDA 2011
-
- NIDA Avant‐Garde‐Medications Development Award winners announced: Grants support novel approaches for treating methamphetamine and nicotine addiction. http://m.drugabuse.gov/news‐events/news‐releases/2011/09/nida‐avant‐gard... (accessed 29 March 2012) 20 September 2011.
Ottney 2011
-
- Ottney AR. Nicotine conjugate vaccine as a novel approach to smoking cessation. Pharmacotherapy 2011;31(7):703‐13. - PubMed
Pentel 2000
-
- Pentel PR, Malin DH, Ennifar S, Hieda Y, Keyler DE, Lake JR, et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacology Biochemistry and Behavior 2000;65(1):191‐8. - PubMed
Pentel 2004
-
- Pentel PR. Vaccines and depot medication for drug addiction: rationale, mechanisms of action, and treatment implications. In: Harwood H, Myers T editor(s). New treatments for addiction: behavioural, ethical, legal and social questions. Washingto DC: National Academies Press, 2004. - PubMed
Quenqua 2011
-
- Quenqua D. An Addiction Vaccine, Tantalizingly Close. New York Times 2011:http://www.nytimes.com/2011/10/04/health/04vaccine.html?pagewanted=all (accessed 29 March 2012).
Raupach 2012
Scherer 1999
-
- Scherer G. Smoking behavior and compensation: a review of the literature. Psychopharmacology 1999;145:1‐20. - PubMed
Scripps 2011
-
- The Scripps Research Institute. Scientists Create Vaccine Against Heroin High. News & Views: Online weekly of the Scripps Research Institute 2011; Vol. 11, issue 23:http://www.scripps.edu/newsandviews/e_20110801/heroin.html (accessed 29 March 2012).
Selecta 2011
-
- Selecta Biosciences. Selecta Biosciences Initiates Phase 1 Clinical Study of SEL‐068, a First‐in‐Class Synthetic Nicotine Vaccine for Smoking Cessation and Relapse Prevention. http://www.selectabio.com/news/recent‐news/Selecta‐Biosciences‐Initiates... (accessed 15 March 2012) 2011.
Siu 2007
-
- Siu ECK, Tyndale RF. Non‐nicotinic therapies for smoking cessation. Annual Review of Pharmacology and Toxicology 2007;47:541‐64. - PubMed
Stead 2005
Stead 2006
Stead 2008a
Stead 2008b
Stolerman 1995
-
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2‐10; 14‐20. - PubMed
WHO 2006
-
- World Health Organization. WHO Framework Convention on Tobacco Control: why is it important?. http://www.who.int/features/qa/34/en/print.html (accessed 24th November 2007) 2006.
WHO 2011
-
- World Health Organization Tobacco Free Initiative. Tobacco Facts. http://www.who.int/tobacco/mpower/tobacco_facts/en/index.html (accessed 12 March 2012) 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

