Nutritional interventions for liver-transplanted patients
- PMID: 22895962
- PMCID: PMC11787929
- DOI: 10.1002/14651858.CD007605.pub2
Nutritional interventions for liver-transplanted patients
Abstract
Background: Malnutrition is a common problem for patients waiting for orthotopic liver transplantation and a risk factor for post-transplant morbidity. The decision to initiate enteral or parenteral nutrition, to which patients and at which time, is still debated. The effects of nutritional supplements given before or after liver transplantation, or both, still remains unclear.
Objectives: The aim of this review was to assess the beneficial and harmful effects of enteral and parenteral nutrition as well as oral nutritional supplements administered to patients before and after liver transplantation.
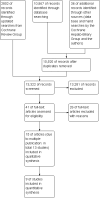
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register (March 2012), the Cochrane Central Register of Controlled Trials (Issue 2 of 12, 2012) in The Cochrane Library, MEDLINE (January 1946 to March 2012), EMBASE (January 1974 to March 2012), Science Citation Index Expanded (January 1900 to March 2012), Social Science Citation Index (January 1961 to October 2010), and reference lists of articles. Manufacturers and experts in the field have also been contacted and relevant journals and conference proceedings were handsearched (from 1997 to October 2010).
Selection criteria: Randomised clinical trials of parallel or cross-over design evaluating the beneficial or harmful effects of enteral or parenteral nutrition or oral nutritional supplements for patients before and after liver transplantation were eligible for inclusion.
Data collection and analysis: Two authors independently assessed the risk of bias of the trials and extracted data. Dichotomous data were reported as odds ratios (OR) and continuous data as mean differences (MD) along with their corresponding 95% confidence intervals (CI). Meta-analysis was not possible due to clinical heterogeneity of included interventions.
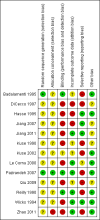
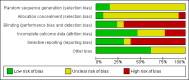
Main results: Thirteen trials met the inclusion criteria. Four publications did not report outcomes pre-defined in the review protocol, or other clinically relevant outcomes and additional data could not be obtained. Nine trials could provide data for the review. Most of the 13 included trials were small and at high risk of bias. Meta-analyses were not possible due to clinical heterogeneity of the interventions.No interventions that were likely to be beneficial were identified.For interventions of unknown effectiveness,postoperative enteral nutrition compared with postoperative parenteral nutrition seemed to have no beneficial or harmful effects on clinical outcomes. Parenteral nutrition containing protein, fat, carbohydrates, and branched-chain amino acids with or without alanyl-glutamine seemed to have no beneficial effect on the outcomes of one and three years survival when compared with a solution of 5% dextrose and normal saline. Enteral immunonutrition with Supportan® seemed to have no effect on occurrence of immunological rejection when compared with enteral nutrition with Fresubin®.There is weak evidence that, compared with standard dietary advice, adding a nutritional supplement to usual diet for patients during the waiting time for liver transplantation had an effect on clinical outcomes after liver transplantation. The combination of enteral nutrition plus parenteral nutrition plus glutamine-dipeptide seemed to be beneficial in reducing length of hospital stay after liver transplantation compared with standard parenteral nutrition (mean difference (MD) -12.20 days; 95% CI -20.20 to -4.00). There is weak evidence that the use of parenteral nutrition plus branched-chain amino acids had an effect on clinical outcomes compared with standard parenteral nutrition, but each was beneficial in reducing length of stay in intensive care unit compared to a standard glucose solution (MD -2.40; 95% CI -4.29 to -0.51 and MD -2.20 days; 95% CI -3.79 to -0.61). There is weak evidence that adding omega-3 fish oil to parenteral nutrition reduced the length of hospital stay after liver transplantation (mean difference -7.1 days; 95% CI -13.02 to -1.18) and the length of stay in intensive care unit after liver transplantation (MD -1.9 days; 95% CI -1.9 to -0.22).For interventions unlikely to be beneficial, there is a significant increased risk in acute rejections in malnourished patients with a history of encephalopathy and treated with the nutritional supplement Ensure® compared with usual diet only (MD 0.70 events per patient; 95% CI 0.08 to 1.32).
Authors' conclusions: We were unable to identify nutritional interventions for liver transplanted patients that seemed to offer convincing benefits. Further randomised clinical trials with low risk of bias and powerful sample sizes are needed.
Conflict of interest statement
None known
Figures
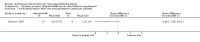
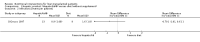



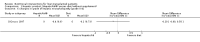














































Update of
- doi: 10.1002/14651858.CD007605
References
References to studies included in this review
Badalamenti 1995 {published data only}
-
- Badalamenti S, Salerno F, Lorenzano E, Paone G, Como G, Finazzi S, et al. Renal effects of dietary supplementation with fish oil in cyclosporine‐treated liver transplant recipients. Hepatology 1995;22(6):1695‐701. - PubMed
DiCecco 1997 {published data only}
-
- Cecco SR, Francisco‐Ziller NM, Porayko MK, Crippin JS, Blue LS, Huang KS, et al. Does pretransplant oral nutrition supplementation affect post‐transplant clinical outcomes? [abstract]. Hepatology 1997;26(4 (Pt 2)):500A.
-
- Hasse J, Crippin J, Blue L, Huang K, DiCecco S, Francisco‐Ziller N, et al. Does nutrition supplementation benefit liver transplant candidates with a history of encephalopathy?. JPEN. Journal of Parenteral and Enteral Nutrition 1997;21(1):S16.
Hasse 1995 {published data only}
-
- Hasse JM, Blue LS, Liepa GU, Goldstein RM, Jennings LW, Mor E, et al. Early enteral nutrition support in patients undergoing liver transplantation. JPEN. Journal of Parenteral and Enteral Nutrition 1995;19(6):437‐43. - PubMed
Jiang 2007 {published data only}
-
- Jiang H, Li B, Yan LN, Lu SC, Wen TF, Zhao JC, et al. Effect of Intravenous glutamine‐dipeptide fortified enteral nutrition on clinical outcomes in patients after liver transplantation: A prospective randomized controlled study. Chinese Journal of Clinical Nutrition 2007;15(1):21‐5.
Jiang 2011 {published data only}
-
- Jiang T, Wang X, Yang AZ, Lu L, Zhang DH, Zhang RS. Effects of omega‐3 fish oil emulsion on changes in serum cytokines after liver transplantation. Journal of Clinical Rehabilitative Tissue Engineering Research 2011;15(31):5726‐30.
Kuse 1990 {published data only}
-
- Kuse ER, Kemnitz J, Kotzerke J, Wassmann R, Gubernatis G, Ringe B, et al. Fat emulsions in parenteral nutrition after liver transplantation: The recovery of the allografts RES function and histological observations. Clinical Nutrition 1990;9(6):331‐6. - PubMed
-
- Kuse ER, Kotzerke J, Ringe B, Wassmann R, Gubernatis G, Pichlmayr I. Fat emulsions in parenteral feeding following liver transplantation. Effect on the recovery of RES function in the transplant [Fettemulsionen in der parenteralen Ernährung nach Lebertransplantation]. Anästhesie, Intensivtherapie, Notfallmedizin 1990;25(6):428‐31. - PubMed
Kuse 2002 {published data only}
-
- Kuse ER, Kotzerke J, Muller S, Nashan B, Luck R, Jaeger K. Hepatic reticuloendothelial function during parenteral nutrition including an MCT/LCT or LCT emulsion after liver transplantation ‐ a double‐blind study. Transplant International 2002;15(6):272‐7. - PubMed
Le Cornu 2000 {published data only}
-
- Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation 2000;69(7):1364‐9. - PubMed
Pazirandeh 2007 {published data only}
Qiu 2009 {published data only}
-
- Qiu Y, Zhu X, Wang W, Xu Q, Ding Y. Nutrition support with glutamine dipeptide in patients undergoing liver transplantation. Transplantation Proceedings 2009;41(10):4232‐7. - PubMed
Reilly 1990 {published data only}
-
- Reilly J, Mehta R, Teperman L, Cemaj S, Tzakis A, Yanaga K, et al. Nutritional support after liver transplantation: a randomized prospective study. JPEN. Journal of Parenteral and Enteral Nutrition 1990;14(4):386‐91. - PubMed
Wicks 1994 {published data only}
-
- Wicks C, Somasundaram S, Bjarnason I, Menzies I S, Routley D, Potter D, et al. Comparison of enteral feeding and total parenteral nutrition after liver transplantation. Lancet 1994;344(8926):837‐40. - PubMed
Zhao 2011 {published data only}
-
- Zhao DF, Zhang K, Lang R, Zhao LJ. Clinical observation of enteral immunonutrition in patients undergoing liver transplantation. Journal of Clinical Rehabilitative Tissue Engeineering Research 2011;15(31):5873‐8.
References to studies excluded from this review
Atamaz 2006 {published data only}
-
- Atamaz F, Hepguler S, Akyildiz M, Karasu Z, Kilic M. Effects of alendronate on bone mineral density and bone metabolic markers in patients with liver transplantation. Osteoporosis International 2006;17(6):942‐9. - PubMed
Atamaz 2006a {published data only}
-
- Atamaz F, Hepguler S, Karasu Z, Kilic M, Tokat Y. The prevention of bone fractures after liver transplantation: experience with alendronate treatment. Transplantation Proceedings 2006;38(5):1448‐52. - PubMed
Bodingbauer 2007 {published data only}
-
- Bodingbauer M, Wekerle T, Pakrah B, Roschger P, Peck‐Radosavljevic M, Silberhumer G, et al. Prophylactic bisphosphonate treatment prevents bone fractures after liver transplantation. American Journal of Transplantation 2007;7(7):1763‐9. - PubMed
Boudreaux 1993 {published data only}
-
- Boudreaux JP, Hayes DH, Mizrahi S, Maggiore P, Blazek J, Dick D. Use of water‐soluble liquid vitamin E to enhance cyclosporine absorption in children after liver transplant. Transplantation Proceedings 1993;25(2):1875. - PubMed
Chin 1990 {published data only}
-
- Chin SE, Shepherd RW, Cleghorn GJ, Patrick M, Ong TH, Wilcox J, et al. Pre‐operative nutritional support in children with end‐stage liver disease accepted for liver transplantation: an approach to management. Journal of Gastroenterology and Hepatology 1990;5(5):566‐72. - PubMed
Chin 1992 {published data only}
-
- Chin SE, Shepherd RW, Thomas BJ, Cleghorn GJ, Patrick MK, Wilcox JA, et al. Nutritional support in children with end‐stage liver disease: a randomized crossover trial of a branched‐chain amino acid supplement. The American Journal of Clinical Nutrition 1992;56(1):158‐63. - PubMed
Clarke 2004 {published data only}
-
- Clarke S, Bernal W, Rees G, Wendon J. Immunonutrition in critically ill patients with liver disease; a prospective randomised double‐blind controlled trial. Hepatology 2004;40 Suppl 1(4):500A.
Crawford 2006 {published data only}
-
- Crawford BA, Kam C, Pavlovic J, Byth K, Handelsman DJ, Angus PW, et al. Zoledronic acid prevents bone loss after liver transplantation: a randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 2006;144(4):239‐48. - PubMed
Eguchi 2011 {published data only}
-
- Eguchi S, Takatsuki M, Hidaka M, Soyama A, Ichikawa T, Kanematsu T. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: a prospective randomized study. American Journal of Surgery 2011;201(4):498‐502. - PubMed
Fulton 1989 {published data only}
-
- Fulton JD. Lactulose as an anti‐endotoxin in liver transplantation. Lancet 1989;2(8668):927‐8. - PubMed
Guy 1995 {published data only}
-
- Guy S, Tanzer‐Torres G, Palese M, Sheiner P, Mor E, Emre S, et al. Does nasoenteral nutritional support reduce mortality after liver transplant?. Hepatology 1995;22:114A.
Hay 2001 {published data only}
-
- Hay JE, Malinchoc M, Dickson ER. A controlled trial of calcitonin therapy for the prevention of post‐liver transplantation atraumatic fractures in patients with primary biliary cirrhosis and primary sclerosing cholangitis. Journal of Hepatology 2001;34(2):292‐8. - PubMed
Hay 2008 {published data only}
-
- Hay JE. How effective is bisphosphonate treatment for preventing bone fractures after liver transplantation?. Nature Clinical Practice. Gastroenterology & Hepatology 2008;5(4):190‐1. - PubMed
Hommann 2007 {published data only}
-
- Hommann M, Lehmann G, Kaemmerer D, Wolf G, Settmacher U. Osteoporosis after liver transplantation ‐ How to prevent fractures? A prospective randomized trial. Liver Transplantation 2007;13 Suppl 1(6).
Kaemmerer 2010 {published data only}
-
- Kaemmerer D, Lehmann G, Wolf G, Settmacher U, Hommann M. Treatment of osteoporosis after liver transplantation with ibandronate. Transplant International 2010;23(7):753‐9. - PubMed
Kim 2011 {published data only}
Krasnoff 2006 {published data only}
-
- Krasnoff JB, Vintro AQ, Ascher NL, Bass NM, Paul SM, Dodd MJ, et al. A randomized trial of exercise and dietary counselling after liver transplantation. American Journal of Transplantation 2006;6(8):1896‐905. - PubMed
Kuse 1991 {published data only}
-
- Kuse ER, Wassmann RM, Ringe B, Bunzendahl H, Lehmkuhl P, Pichlmayr I, et al. Fatty emulsions in parenteral feeding following liver transplantation. A study of the neurotropic effect of MCT/LCT emulsions using EEG [Fettemulsionen in der parenteralen Ernährung nach Lebertransplantation]. Anästhesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 1991;26(2):96‐101. - PubMed
Mager 2006 {published data only}
-
- Mager DR, Wykes LJ, Roberts EA, Ball RO, Pencharz PB. Effect of orthotopic liver transplantation (OLT) on branched‐chain amino acid requirement. Pediatric Research 2006;59(6):829‐34. - PubMed
Neuhaus 1995 {published data only}
-
- Neuhaus R, Lohmann R, Platz KP, Guckelberger O, Schon M, Lang M, et al. Treatment of osteoporosis after liver transplantation. Transplantation Proceedings 1995;27(1):1226‐7. - PubMed
Neuhaus 1999 {published data only}
-
- Neuhaus R, Kubo A, Lohmann R, Rayes N, Hierholzer J, Neuhaus P. Calcitriol in prevention and therapy of osteoporosis after liver transplantation. Transplantation Proceedings 1999;31(1‐2):472‐3. - PubMed
Nickkholgh 2007 {published data only}
Perkins 2006 {published data only}
-
- Perkins JD. Bone loss after liver transplantation: A randomized, double‐blind, placebo‐controlled trial. Liver Transplantation 2006;12(7):1168‐9. - PubMed
Ramaccioni 2000 {published data only}
-
- Ramaccioni V, Soriano HE, Arumugam R, Klish WJ. Nutritional aspects of chronic liver disease and liver transplantation in children. Journal of Pediatric Gastroenterology and Nutrition 2000;30(4):361‐7. - PubMed
Riordan 1999 {published data only}
-
- Riordan SM, Williams R. Nutrition and liver transplantation. Journal of Hepatology 1999;31(5):955‐62. - PubMed
Ukleja 2002 {published data only}
-
- Ukleja A, Scolapio JS, McConnell JP, Spivey JR, Dickson RC, Nguyen JH, et al. Nutritional assessment of serum and hepatic vitamin A levels in patients with cirrhosis. JPEN. Journal of Parenteral and Enteral Nutrition 2002;26(3):184‐8. - PubMed
Valero 1995 {published data only}
-
- Valero MA, Loinaz C, Larrodera L, Leon M, Moreno E, Hawkins F. Calcitonin and bisphosphonates treatment in bone loss after liver transplantation. Calcified Tissue International 1995;57(1):15‐9. - PubMed
References to ongoing studies
Luntz 2005 {published data only}
-
- Luntz SP, Unnebrink K, Seibert‐Grafe M, Bunzendahl H, Kraus TW, Büchler MW, et al. HEGPOL: Randomized, placebo controlled, multicenter,double‐blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation [ISRCTN69350312]. BMC Surgery 2005;5:18. - PMC - PubMed
Additional references
A.S.P.E.N. 2012
-
- American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors and Clinical Practice Committee. Definition of Terms, Style, and Conventions Used in A.S.P.E.N. Board of Directors. http://www.nutritioncare.org/wcontent.aspx?id=4714s 2012 (accessed 26. June 2012).
Aranda‐Michel 2001
-
- Aranda‐Michel J. Nutrition in hepatic failure and liver transplantation. Current Gastroenterology Reports 2001;3(4):362‐70. - PubMed
Baker 2007
-
- Baker A, Stevenson R, Dhawan A, Goncalves I, Socha P, Sokal E. Guidelines for nutritional care for infants with cholestatic liver disease before liver transplantation. Pediatric Transplantation 2007;11(8):825‐34. - PubMed
Campos 2002
-
- Campos AC, Matias JE, Coelho JC. Nutritional aspects of liver transplantation. Current Opinion in Clinical Nutrition and Metabolic Care 2002;5(3):297‐307. - PubMed
Cunha 2004
-
- Cunha L, Happi Nono M, Guibert AL, Nidegger D, Beau P, Beauchant M. Effects of prolonged oral nutritional support in malnourished cirrhotic patients: results of a pilot study. Gastroenterologie Clinique et Biologique 2004;28(1):36‐9. [0399‐8320 (Print)] - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
DiCenso 2005
-
- DiCenso A, Guyatt G, Ciliska D. Evidence‐Based Nursing. A Guide to Clinical Practice. St. Louis: Elsevier Mosby, 2005.
Egger 1997
Egger 2001
-
- Egger M, Smith DE, Altmann DG. Systematic Reviews in Health Care: Meta‐Analysis in Context. Second Edition. London: BMJ Publishing Group, 2001.
Eurotransplant 2010
-
- Eurotransplant. Eurotransplant. Annual Report 2010. http://www.eurotransplant.org/cms/mediaobject.php?file=ar_2010.pdf 2010 (accessed 26. June 2012).
Ferreira 2010
-
- Ferreira LG, Anastácio LR, Correia MI. The impact of nutrition on cirrhotic patients awaiting liver transplantation. Current Opinion in Clinical Nutrition and Metabolic Care 2010;13(5):554‐61. - PubMed
Gluud 2012
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2012, Issue 5. Art. No.: LIVER.
Grungreiff 2005
-
- Grungreiff K, Reinhold D. Liver cirrhosis and "liver" diabetes mellitus are linked by zinc deficiency. Medical Hypotheses 2005;64(2):316‐7. - PubMed
Hasse 1995a
-
- Hasse JM, Blue LS, Liepa GU, Goldstein RM, Jennings LW, Mor E, et al. Early enteral nutrition support in patients undergoing liver transplantation. JPEN. Journal of Parenteral and Enteral Nutrition 1995;19(6):437‐43. - PubMed
Hasse 2006
-
- Hasse JM. Examining the role of tube feeding after liver transplantation. Nutrition in Clinical Practice 2006;21(3):299‐311. - PubMed
Henkel 2006
-
- Henkel AS, Buchman AL. Nutritional support in patients with chronic liver disease. Nature Clinical Practice. Gastroenterology & Hepatology 2006;3(4):202‐9. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsch 1993
-
- Hirsch S, Bunout D, Maza P, Iturriaga H, Petermann M, Icazar G, et al. Controlled trial on nutrition supplementation in outpatients with symptomatic alcoholic cirrhosis. JPEN. Journal of Parenteral and Enteral Nutrition 1993;17(2):119‐24. - PubMed
Khanna 2007
-
- Khanna S, Gopalan S. Role of branched‐chain amino acids in liver disease: the evidence for and against. Current Opinion in Clinical Nutrition and Metabolic Care 2007;10(3):297‐303. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Koretz 2001
-
- Koretz RL, Lipman, TO, Klein S. AGA technical review on parenteral nutrition. Gastroenterology 2001;121(4):970‐1001. - PubMed
Koretz 2007
-
- Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. American Journal of Gastroenterology 2007;102(2):412‐29. - PubMed
Kunz 2007
Le Cornu 2000a
-
- Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation 2000;69(7):1364‐9. - PubMed
Lochs 2006
-
- H. Lochs H, Allison SP, Meier R, Pirlich M, Kondrup J, Schneider S, et al. Introductory to the ESPEN Guidelines on Enteral Nutrition: terminology, definitions and general topics. Clinical Nutrition 2006;25:180‐6. - PubMed
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Marchesini 1996
-
- Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M. Zinc supplementation and amino acid‐nitrogen metabolism in patients with advanced cirrhosis. Hepatology 1996;23(5):1084‐92. - PubMed
Marchesini 2003
-
- Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, et al. Nutritional supplementation with branched‐chain amino acids in advanced cirrhosis: a double‐blind, randomized trial. Gastroenterology 2003;124(7):1792‐801. - PubMed
Matos 2002
-
- Matos C, Porayko MK, Francisco‐Ziller N, DiCecco S. Nutrition and chronic liver disease. Journal of Clinical Gastroenterology 2002;35(5):391‐7. [0192‐0790 (Print)] - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Muto 2005
-
- Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, et al. Effects of oral branched‐chain amino acid granules on event‐free survival in patients with liver cirrhosis. Clinical Gastroenterology and Hepatology 2005;3(7):705‐13. - PubMed
O'Brien 2008
-
- O'Brien A, Williams R. Nutrition in end‐stage liver disease: principles and practice. Gastroenterology 2008;134(6):1729‐40. - PubMed
Plank 2005
-
- Plank LD, McCall JL, Gane EJ, Rafique M, Gillanders LK, McIlroy K, et al. Pre‐ and postoperative immunonutrition in patients undergoing liver transplantation: a pilot study of safety and efficacy. Clinical Nutrition 2005;24(2):288‐96. - PubMed
Plauth 1997
-
- Plauth M, Merli M, Kondrup J, Weimann A, Ferenci P, Muller MJ. ESPEN guidelines for nutrition in liver disease and transplantation. Clinical Nutrition 1997;16(2):43‐55. - PubMed
Plauth 2009
Poon 2004
-
- Poon RT, Yu WC, Fan ST, Wong J. Long‐term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Alimentary Pharmacology and Therapeutics 2004;19(7):779‐88. - PubMed
Porta 2007
-
- Porta N, Bonet C, Cobo E. Discordance between reported intention‐to‐treat and per protocol analyses. Journal of Clinical Epidemiology 2007;60:663‐9. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Rücker 2007
-
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2007;27(5):746‐63. - PubMed
Sanchez 2006
-
- Sanchez AJ, Aranda‐Michel J. Nutrition for the liver transplant patient. Liver Transplantation 2006;12(9):1310‐6. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Stickel 2008
-
- Stickel F, Inderbitzin D, Candinas D. Role of nutrition in liver transplantation for end‐stage chronic liver disease. Nutrition Reviews 2008;66(1):47‐54. - PubMed
Thuluvath 2010
-
- Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999‐2008. American Journal of Transplantation 2010;10(4 Pt 2):1003‐19. - PubMed
Vintro 2002
-
- Vintro AQ, Krasnoff JB, Painter P. Roles of nutrition and physical activity in musculoskeletal complications before and after liver transplantation. AACN Clinical Issues 2002;13(2):333‐47. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

