Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation
- PMID: 22896000
- PMCID: PMC4916583
- DOI: 10.1182/blood-2012-03-415307
Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation
Abstract
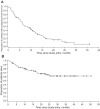
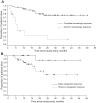
Chronic myeloid leukemia (CML) patients with the BCR-ABL T315I mutation do not benefit from therapy with currently approved tyrosine kinase inhibitors. Omacetaxine mepesuccinate is a protein synthesis inhibitor that has demonstrated activity in cells harboring the T315I mutation. This phase 2 trial assessed the efficacy of omacetaxine in CML patients with T315I and tyrosine kinase inhibitor failure. Patients received subcutaneous omacetaxine 1.25 mg/m(2) twice daily, days 1-14, every 28 days until hematologic response or a maximum of 6 cycles, and then days 1-7 every 28 days as maintenance. Results for patients treated in chronic phase are reported here. Patients (n = 62) received a median of 7 (range, 1-41) cycles. Complete hematologic response was achieved in 48 patients (77%; 95% lower confidence limit, 65%); median response duration was 9.1 months. Fourteen patients (23%; 95% lower confidence limit, 13%) achieved major cytogenetic response, including complete cytogenetic response in 10 (16%). Median progression free-survival was 7.7 months. Grade 3/4 hematologic toxicity included thrombocytopenia (76%), neutropenia (44%), and anemia (39%) and was typically manageable by dose reduction. Nonhematologic adverse events were mostly grade 1/2 and included infection (42%), diarrhea (40%), and nausea (34%). Omacetaxine may provide a safe and effective treatment for CML patients with T315I mutation. This study is registered at www.clinicaltrials.gov as NCT00375219.
Figures
References
-
- Gambacorti-Passerini C, Antolini L, Mahon FX, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553–561. - PubMed
-
- Hochhaus A, O'Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–1061. - PubMed
-
- Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. - PubMed
-
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. - PubMed
-
- Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosome-positive leukaemias. Lancet Oncol. 2003;4(2):75–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

