Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells
- PMID: 22896036
- PMCID: PMC3517696
- DOI: 10.1158/2159-8290.CD-12-0216
Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells
Abstract
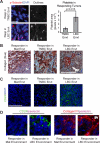
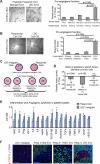
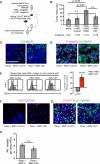
Breast cancer recurrence rates vary following treatment, suggesting that tumor cells disseminate early from primary sites but remain indolent indefinitely before progressing to symptomatic disease. The reasons why some indolent disseminated tumors erupt into overt disease are unknown. We discovered a novel process by which certain luminal breast cancer (LBC) cells and patient tumor specimens (LBC "instigators") establish a systemic macroenvironment that supports outgrowth of otherwise-indolent disseminated tumors ("responders"). Instigating LBCs secrete cytokines that are absorbed by platelets, which are recruited to responding tumor sites where they aid vessel formation. Instigator-activated bone marrow cells enrich responding tumor cell expression of CD24, an adhesion molecule for platelets, and provide a source of VEGF receptor 2(+) tumor vessel cells. This cascade results in growth of responder adenocarcinomas and is abolished when platelet activation is inhibited by aspirin. These findings highlight the macroenvironment as an important component of disease progression that can be exploited therapeutically.
Significance: Currently, processes that mediate progression of otherwise indolent tumors are not well understood, making it difficult to accurately predict which cancer patients are likely to relapse. Our findings highlight the macroenvironment as an important component of disease progression that can be exploited to more accurately identify patients who would benefit from adjuvant therapy.
©2012 AACR.
Figures




Comment in
-
Hello out there...is anybody listening?Cancer Discov. 2012 Dec;2(12):1084-6. doi: 10.1158/2159-8290.CD-12-0434. Cancer Discov. 2012. PMID: 23230186 Free PMC article.
References
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. - PubMed
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. - PubMed
-
- Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2011;133:831–41. - PubMed
-
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

