Aggregation by depletion attraction in cultures of bacteria producing exopolysaccharide
- PMID: 22896568
- PMCID: PMC3481587
- DOI: 10.1098/rsif.2012.0498
Aggregation by depletion attraction in cultures of bacteria producing exopolysaccharide
Abstract
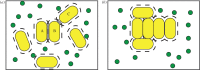
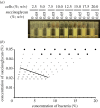
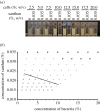
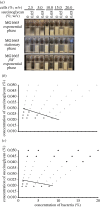
In bacteria, the production of exopolysaccharides--polysaccharides secreted by the cells into their growth medium--is integral to the formation of aggregates and biofilms. These exopolysaccharides often form part of a matrix that holds the cells together. Investigating the bacterium Sinorhizobium meliloti, we found that a mutant that overproduces the exopolysaccharide succinoglycan showed enhanced aggregation, resulting in phase separation of the cultures. However, the aggregates did not appear to be covered in polysaccharides. Succinoglycan purified from cultures was applied to different concentrations of cells, and observation of the phase behaviour showed that the limiting polymer concentration for aggregation and phase separation to occur decreased with increasing cell concentration, suggesting a 'crowding mechanism' was occurring. We suggest that, as found in colloidal dispersions, the presence of a non-adsorbing polymer in the form of the exopolysaccharide succinoglycan drives aggregation of S. meliloti by depletion attraction. This force leads to self-organization of the bacteria into small clusters of laterally aligned cells, and, furthermore, leads to aggregates clustering into biofilm-like structures on a surface.
Figures




References
-
- Deinema M. H., Zevenhuizen L. P. 1971. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch. Mikrobiol. 78, 42–51 10.1007/BF00409087 (doi:10.1007/BF00409087) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
