Cellular mechanisms of aortic valve calcification
- PMID: 22896576
- PMCID: PMC3427002
- DOI: 10.1161/CIRCINTERVENTIONS.112.971028
Cellular mechanisms of aortic valve calcification
Abstract
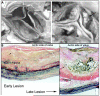
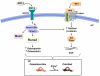
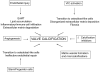
Acquired aortic valve disease and valvular calcification is highly prevalent in adult populations worldwide and is associated with significant cardiovascular morbidity and mortality. At present, there are no medical therapies that will prevent or regress aortic valve calcification or stenosis and surgical or transcatheter aortic valve replacement remain the only effective therapies for treating this disease. In the setting of valve injury as a result of exposure to biochemical mediators or hemodynamic forces, normal homeostatic processes are disrupted resulting in extracellular matrix degradation, aberrant matrix deposition and fibrosis, inflammatory cell infiltration, lipid accumulation, and neoangiogenesis of the valve tissue and, ultimately, calcification of the valve. Calcification of the aortic valve is now understood to be an active process that involves the coordinated actions of resident valve endothelial and interstitial cells, circulating inflammatory and immune cells, and bone marrow-derived cells. These cells may undergo a phenotype transition to become osteoblast-like cells and elaborate bone matrix, endothelial-to-mesenchymal transition, and form matrix vesicles that serve as a nidus for microcalcifications. Each of these mechanisms has been shown to contribute to aortic valve calcification suggesting that strategies that target these cellular events may lead to novel therapeutic interventions to halt the progression or reverse aortic valve calcification.
Figures


References
-
- Vaslef SN, Roberts WC. Early descriptions of aortic valve stenosis. Am Heart J. 1993;125:1465–1474. - PubMed
-
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. - PMC - PubMed
-
- Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. - PubMed
-
- Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. - PubMed
-
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

