Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection
- PMID: 22896601
- PMCID: PMC3486289
- DOI: 10.1128/JVI.00615-12
Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection
Abstract
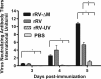
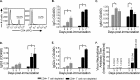
A major goal in rabies virus (RV) research is to develop a single-dose postexposure prophylaxis (PEP) that would simplify vaccination protocols, reduce costs associated with rabies prevention in humans, and save lives. Live replication-deficient RV-based vaccines are emerging as promising single-dose vaccines to replace currently licensed inactivated RV-based vaccines. Nonetheless, little is known about how effective B cells develop in response to live RV-based vaccination. Understanding this fundamental property of rabies immunology may help in developing a single-dose RV vaccine. Typically, vaccines induce B cells secreting high-affinity, class-switched antibodies during germinal center (GC) reactions; however, there is a lag time between vaccination and the generation of GC B cells. In this report, we show that RV-specific antibodies are detected in mice immunized with live but not inactivated RV-based vaccines before B cells displaying a GC B cell phenotype (B220(+)GL7(hi)CD95(hi)) are formed, indicating a potential role for T cell-independent and early extrafollicular T cell-dependent antibody responses in the protection against RV infection. Using two mouse models of CD4(+) T cell deficiency, we show that B cells secreting virus-neutralizing antibodies (VNAs) are induced via T cell-independent mechanisms within 4 days postimmunization with a replication-deficient RV-based vaccine. Importantly, mice that are completely devoid of T cells (B6.129P2-Tcrβ(tm1Mom) Tcrδ(tm1Mom)/J) show protection against pathogenic challenge shortly after immunization with a live replication-deficient RV-based vaccine. We show that vaccines that can exploit early pathways of B cell activation and development may hold the key for the development of a single-dose RV vaccine wherein the rapid induction of VNA is critical.
Figures

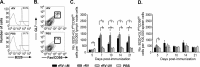


Similar articles
-
Targeting Vaccine-Induced Extrafollicular Pathway of B Cell Differentiation Improves Rabies Postexposure Prophylaxis.J Virol. 2017 Mar 29;91(8):e02435-16. doi: 10.1128/JVI.02435-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148792 Free PMC article.
-
B cell infection and activation by rabies virus-based vaccines.J Virol. 2013 Aug;87(16):9097-110. doi: 10.1128/JVI.00800-13. Epub 2013 Jun 12. J Virol. 2013. PMID: 23760241 Free PMC article.
-
Investigating the role for IL-21 in rabies virus vaccine-induced immunity.PLoS Negl Trop Dis. 2013;7(3):e2129. doi: 10.1371/journal.pntd.0002129. Epub 2013 Mar 14. PLoS Negl Trop Dis. 2013. PMID: 23516660 Free PMC article.
-
Experimental rabies vaccines for humans.Expert Rev Vaccines. 2010 Oct;9(10):1177-86. doi: 10.1586/erv.10.105. Expert Rev Vaccines. 2010. PMID: 20923268 Free PMC article. Review.
-
Rabies Vaccine for Prophylaxis and Treatment of Rabies: A Narrative Review.Cureus. 2024 Jun 15;16(6):e62429. doi: 10.7759/cureus.62429. eCollection 2024 Jun. Cureus. 2024. PMID: 39011185 Free PMC article. Review.
Cited by
-
Safety and serological response to a matrix gene-deleted rabies virus-based vaccine vector in dogs.Vaccine. 2014 Mar 26;32(15):1716-9. doi: 10.1016/j.vaccine.2014.01.043. Epub 2014 Feb 7. Vaccine. 2014. PMID: 24508037 Free PMC article.
-
Rabies vaccination induces a CD4+ TEM and CD4+CD8+ TEMRA TH1 phenotype in dogs.PLoS One. 2025 May 12;20(5):e0323823. doi: 10.1371/journal.pone.0323823. eCollection 2025. PLoS One. 2025. PMID: 40354406 Free PMC article.
-
Overexpression of Interleukin-7 Extends the Humoral Immune Response Induced by Rabies Vaccination.J Virol. 2017 Mar 13;91(7):e02324-16. doi: 10.1128/JVI.02324-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28100620 Free PMC article.
-
Quantitative characterization of the T cell receptor repertoires of human immunized by rabies virus vaccine.Hum Vaccin Immunother. 2021 Aug 3;17(8):2530-2537. doi: 10.1080/21645515.2021.1893575. Epub 2021 Apr 6. Hum Vaccin Immunother. 2021. PMID: 33823121 Free PMC article.
-
Incorporating B cell activating factor (BAFF) into the membrane of rabies virus (RABV) particles improves the speed and magnitude of vaccine-induced antibody responses.PLoS Negl Trop Dis. 2019 Nov 14;13(11):e0007800. doi: 10.1371/journal.pntd.0007800. eCollection 2019 Nov. PLoS Negl Trop Dis. 2019. PMID: 31725816 Free PMC article.
References
-
- Bunschoten H, et al. 1990. Rabies virus cross-reactive murine T cell clones: analysis of helper and delayed-type hypersensitivity function. Viral Immunol. 3:41–53 - PubMed
-
- Faul EJ, et al. 2010. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog. 6:e1001016 doi:10.1371/journal.ppat.1001016 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

