Oral clarithromycin enhances airway immunoglobulin A (IgA) immunity through induction of IgA class switching recombination and B-cell-activating factor of the tumor necrosis factor family molecule on mucosal dendritic cells in mice infected with influenza A virus
- PMID: 22896605
- PMCID: PMC3457149
- DOI: 10.1128/JVI.01207-12
Oral clarithromycin enhances airway immunoglobulin A (IgA) immunity through induction of IgA class switching recombination and B-cell-activating factor of the tumor necrosis factor family molecule on mucosal dendritic cells in mice infected with influenza A virus
Abstract
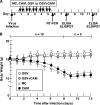
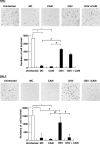
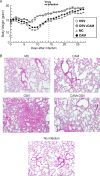
We previously reported that the macrolide antibiotic clarithromycin (CAM) enhanced the mucosal immune response in pediatric influenza, particularly in children treated with the antiviral neuraminidase inhibitor oseltamivir (OSV) with low production of mucosal antiviral secretory IgA (S-IgA). The aims of the present study were to confirm the effects of CAM on S-IgA immune responses, by using influenza A virus (IAV) H1N1-infected mice treated with or without OSV, and to determine the molecular mechanisms responsible for the induction of mucosal IgA class switching recombination in IAV-infected CAM-treated mice. The anti-IAV S-IgA responses and expression levels of IgA class switching recombination-associated molecules were examined in bronchus-lymphoid tissues and spleens of infected mice. We also assessed neutralization activities of S-IgA against IAV. Data show that CAM enhanced anti-IAV S-IgA induction in the airway of infected mice and restored the attenuated antiviral S-IgA levels in OSV-treated mice to the levels in the vehicle-treated mice. The expression levels of B-cell-activating factor of the tumor necrosis factor family (BAFF) molecule on mucosal dendritic cells as well as those of activation-induced cytidine deaminase and Iμ-Cα transcripts on B cells were enhanced by CAM, compared with the levels without CAM treatment, but CAM had no effect on the expression of the BAFF receptor on B cells. Enhancement by CAM of neutralization activities of airway S-IgA against IAV in vitro and reinfected mice was observed. This study identifies that CAM enhances S-IgA production and neutralizing activities through the induction of IgA class switching recombination and upregulation of BAFF molecules in mucosal dendritic cells in IAV-infected mice.
Figures





Similar articles
-
Cigarette smoke inhibits BAFF expression and mucosal immunoglobulin A responses in the lung during influenza virus infection.Respir Res. 2015 Mar 14;16(1):37. doi: 10.1186/s12931-015-0201-y. Respir Res. 2015. PMID: 25849069 Free PMC article.
-
Immunomodulator clarithromycin enhances mucosal and systemic immune responses and reduces re-infection rate in pediatric patients with influenza treated with antiviral neuraminidase inhibitors: a retrospective analysis.PLoS One. 2013 Jul 17;8(7):e70060. doi: 10.1371/journal.pone.0070060. Print 2013. PLoS One. 2013. PMID: 23875018 Free PMC article.
-
Attenuation of inducible respiratory immune responses by oseltamivir treatment in mice infected with influenza A virus.Microbes Infect. 2010 Sep;12(10):778-83. doi: 10.1016/j.micinf.2010.04.013. Epub 2010 May 7. Microbes Infect. 2010. PMID: 20452454
-
New insights into the pathogenesis of IgA nephropathy: do toll like receptor 9-B cell activation factor-IgA class switching recombination signaling axis induce IgA hyper-production?Ren Fail. 2014 Jul;36(6):970-3. doi: 10.3109/0886022X.2014.916578. Epub 2014 May 15. Ren Fail. 2014. PMID: 24828398 Review.
-
Proteases essential for human influenza virus entry into cells and their inhibitors as potential therapeutic agents.Curr Pharm Des. 2007;13(4):405-14. doi: 10.2174/138161207780162971. Curr Pharm Des. 2007. PMID: 17311557 Review.
Cited by
-
Bakuchiol Is a Phenolic Isoprenoid with Novel Enantiomer-selective Anti-influenza A Virus Activity Involving Nrf2 Activation.J Biol Chem. 2015 Nov 13;290(46):28001-17. doi: 10.1074/jbc.M115.669465. Epub 2015 Oct 7. J Biol Chem. 2015. PMID: 26446794 Free PMC article.
-
A novel functional site in the PB2 subunit of influenza A virus essential for acetyl-CoA interaction, RNA polymerase activity, and viral replication.J Biol Chem. 2014 Sep 5;289(36):24980-94. doi: 10.1074/jbc.M114.559708. Epub 2014 Jul 25. J Biol Chem. 2014. PMID: 25063805 Free PMC article.
-
Anti-influenza activity of c60 fullerene derivatives.PLoS One. 2013 Jun 13;8(6):e66337. doi: 10.1371/journal.pone.0066337. Print 2013. PLoS One. 2013. PMID: 23785493 Free PMC article.
-
Clinical evidences on the antiviral properties of macrolide antibiotics in the COVID-19 era and beyond.Antivir Chem Chemother. 2020 Jan-Dec;28:2040206620961712. doi: 10.1177/2040206620961712. Antivir Chem Chemother. 2020. PMID: 32972196 Free PMC article.
-
Cigarette smoke inhibits BAFF expression and mucosal immunoglobulin A responses in the lung during influenza virus infection.Respir Res. 2015 Mar 14;16(1):37. doi: 10.1186/s12931-015-0201-y. Respir Res. 2015. PMID: 25849069 Free PMC article.
References
-
- Al-Garawi AA, et al. 2009. Acute, but not resolved, influenza A infection enhances susceptibility to house dust mite-induced allergic disease. J. Immunol. 182:3095–3104 - PubMed
-
- Barone F, Patel P, Sanderson JD, Spencer J. 2009. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunol. 2:495–503 - PubMed
-
- Brandtzaeg P, Farstad IN, Haraldsen G. 1999. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol. Today 20:267–277 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

