Broadly neutralizing immune responses against hepatitis C virus induced by vectored measles viruses and a recombinant envelope protein booster
- PMID: 22896607
- PMCID: PMC3486281
- DOI: 10.1128/JVI.01776-12
Broadly neutralizing immune responses against hepatitis C virus induced by vectored measles viruses and a recombinant envelope protein booster
Abstract
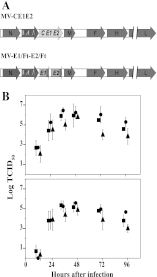
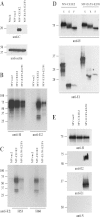
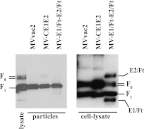
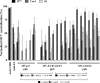
Hepatitis C virus (HCV) infection remains a serious public health problem worldwide. Treatments are limited, and no preventive vaccine is available. Toward developing an HCV vaccine, we engineered two recombinant measles viruses (MVs) expressing structural proteins from the prototypic HCV subtype 1a strain H77. One virus directs the synthesis of the HCV capsid (C) protein and envelope glycoproteins (E1 and E2), which fold properly and form a heterodimer. The other virus expresses the E1 and E2 glycoproteins separately, with each one fused to the cytoplasmic tail of the MV fusion protein. Although these hybrid glycoproteins were transported to the plasma membrane, they were not incorporated into MV particles. Immunization of MV-susceptible, genetically modified mice with either vector induced neutralizing antibodies to MV and HCV. A boost with soluble E2 protein enhanced titers of neutralizing antibody against the homologous HCV envelope. In animals primed with MV expressing properly folded HCV C-E1-E2, boosting also induced cross-neutralizating antibodies against two heterologous HCV strains. These results show that recombinant MVs retain the ability to induce MV-specific humoral immunity while also eliciting HCV neutralizing antibodies, and that anti-HCV immunity can be boosted with a single dose of purified E2 protein. The use of MV vectors could have advantages for pediatric HCV vaccination.
Figures


References
-
- Abraham JD, et al. 2004. Comparative immunogenicity analysis of modified vaccinia Ankara vectors expressing native or modified forms of hepatitis C virus E1 and E2 glycoproteins. Vaccine 22:3917–3928 - PubMed
-
- Amanna IJ, Carlson NE, Slifka MK. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903–1915 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

