Semaphorin 7A contributes to West Nile virus pathogenesis through TGF-β1/Smad6 signaling
- PMID: 22896629
- PMCID: PMC3496209
- DOI: 10.4049/jimmunol.1201140
Semaphorin 7A contributes to West Nile virus pathogenesis through TGF-β1/Smad6 signaling
Abstract
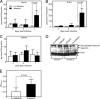
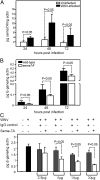
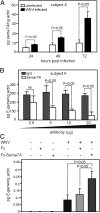
Semaphorin 7A (Sema7A) is a membrane-associated/secreted protein that plays an essential role in connecting the vertebrate neuronal and immune systems. However, the role of Sema7A has not been elucidated in viral pathogenesis. In this study, we show that abrogation of Sema7A protects mice from lethal West Nile virus (WNV) infection. Mice lacking Sema7A showed increased survival, reduced viral burden, and less blood-brain barrier permeability upon WNV infection. Increased Sema7A levels were evident in murine tissues, as well as in murine cortical neurons and primary human macrophages upon WNV infection. Treatment with Sema7A Ab blocked WNV infection in both of these cell types. Furthermore, Sema7A positively regulates the production of TGF-β1 and Smad6 to facilitate WNV pathogenesis in mice. Collectively, these data elucidate the role of Sema7A in shared signaling pathways used by the immune and nervous systems during viral pathogenesis that may lead to the development of Sema7A-blocking therapies for WNV and possibly other flaviviral infections.
Figures



References
-
- Petersen L. R., Marfin A. A. 2002. West Nile virus: a primer for the clinician. Ann. Intern. Med. 137: 173–179 - PubMed
-
- Diamond M. S., Klein R. S. 2004. West Nile virus: crossing the blood-brain barrier. Nat. Med. 10: 1294–1295 - PubMed
-
- Lustig S., Olshevsky U., Ben-Nathan D., Lachmi B. E., Malkinson M., Kobiler D., Halevy M. 2000. A live attenuated West Nile virus strain as a potential veterinary vaccine. Viral Immunol. 13: 401–410 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

