The adaptor 3BP2 is required for early and late events in FcεRI signaling in human mast cells
- PMID: 22896635
- PMCID: PMC3436965
- DOI: 10.4049/jimmunol.1200380
The adaptor 3BP2 is required for early and late events in FcεRI signaling in human mast cells
Abstract
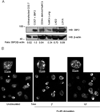
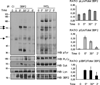
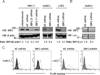
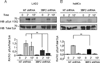
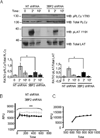
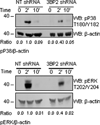
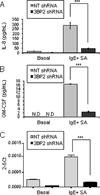
Adaptor molecules are essential in organizing signaling molecules and in coordinating and compartmentalizing their activity. SH3-binding protein 2 (3BP2) is a cytoplasmic adaptor protein mainly expressed by hematopoietic cells that has been shown to act as a positive regulator in T, B, and NK cell signal transduction. 3BP2 is an important regulator of cytotoxic granule release in NK cells. Mast cells (MCs) similarly degranulate following Ag-dependent aggregation of the FcεRI on the cell surface. Activation of these cells induces the release of preformed inflammatory mediators and the de novo synthesis and secretion of cytokines and chemokines. Thus, MCs participate in both innate and acquired responses. We observed that 3BP2 is expressed in human MCs (huMCs) from diverse origins. Moreover, 3BP2 coimmunoprecipitates with essential MC signaling mediators such as Lyn, Syk, and phospholipase C γ; thus, a role for this adaptor in MC function was postulated. In the present work, we used the short hairpin RNA lentiviral targeting approach to silence 3BP2 expression in huMCs. Our findings point to a requirement for 3BP2 in optimal immediate and late MCs responses such as degranulation and IL-8 or GM-CSF secretion. 3BP2 was determined to be necessary for optimal phosphorylation of Syk, linker for activation of T cells, and phospholipase C γ(1), critical signals for calcium release from intracellular stores. Taken together, our results show that by participating in FcεRI- mediated signal transduction 3BP2 is an important regulator of huMC activation. Thus, 3BP2 could be a potential therapeutic target for IgE-dependent MC-mediated inflammatory disease.
Figures

References
-
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. - PubMed
-
- Mekori YA, Oh CK, Metcalfe DD. The role of c-Kit and its ligand, stem cell factor, in mast cell apoptosis. Int Arch Allergy Immunol. 1995;107:136–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

