Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4
- PMID: 22896676
- PMCID: PMC3633234
- DOI: 10.1126/scitranslmed.3003778
Targeted tumor-penetrating siRNA nanocomplexes for credentialing the ovarian cancer oncogene ID4
Erratum in
-
Erratum for the Research Article "Targeted Tumor-Penetrating siRNA Nanocomplexes for Credentialing the Ovarian Cancer Oncogene ID4" by Y. Ren et al.Sci Transl Med. 2024 Mar 27;16(740):eadp2153. doi: 10.1126/scitranslmed.adp2153. Epub 2024 Mar 27. Sci Transl Med. 2024. PMID: 38536938 No abstract available.
Abstract
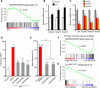
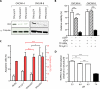
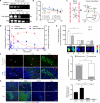
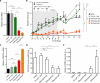
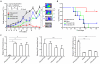
The comprehensive characterization of a large number of cancer genomes will eventually lead to a compendium of genetic alterations in specific cancers. Unfortunately, the number and complexity of identified alterations complicate endeavors to identify biologically relevant mutations critical for tumor maintenance because many of these targets are not amenable to manipulation by small molecules or antibodies. RNA interference provides a direct way to study putative cancer targets; however, specific delivery of therapeutics to the tumor parenchyma remains an intractable problem. We describe a platform for the discovery and initial validation of cancer targets, composed of a systematic effort to identify amplified and essential genes in human cancer cell lines and tumors partnered with a novel modular delivery technology. We developed a tumor-penetrating nanocomplex (TPN) that comprised small interfering RNA (siRNA) complexed with a tandem tumor-penetrating and membrane-translocating peptide, which enabled the specific delivery of siRNA deep into the tumor parenchyma. We used TPN in vivo to evaluate inhibitor of DNA binding 4 (ID4) as a novel oncogene. Treatment of ovarian tumor-bearing mice with ID4-specific TPN suppressed growth of established tumors and significantly improved survival. These observations not only credential ID4 as an oncogene in 32% of high-grade ovarian cancers but also provide a framework for the identification, validation, and understanding of potential therapeutic cancer targets.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA119335/CA/NCI NIH HHS/United States
- U54 CA151884/CA/NCI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- P50 CA105009/CA/NCI NIH HHS/United States
- P30 CA14051/CA/NCI NIH HHS/United States
- U01 CA152990/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U54 CA119349/CA/NCI NIH HHS/United States
- U54 CA112962/CA/NCI NIH HHS/United States
- R33 CA128625/CA/NCI NIH HHS/United States
- CAPMC/ CIHR/Canada
- RC2 CA148268/CA/NCI NIH HHS/United States
- R01 CA121941/CA/NCI NIH HHS/United States
- R01 CA152327/CA/NCI NIH HHS/United States
- R01 CA124427/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

