Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation
- PMID: 22896717
- PMCID: PMC3545183
- DOI: 10.1152/jn.00137.2012
Depolarizing bipolar cell dysfunction due to a Trpm1 point mutation
Abstract
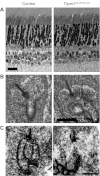
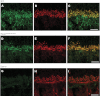
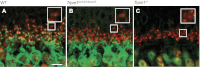
Mutations in TRPM1 are found in humans with an autosomal recessive form of complete congenital stationary night blindness (cCSNB). The Trpm1(-/-) mouse has been an important animal model for this condition. Here we report a new mouse mutant, tvrm27, identified in a chemical mutagenesis screen. Genetic mapping of the no b-wave electroretinogram (ERG) phenotype of tvrm27 localized the mutation to a chromosomal region that included Trpm1. Complementation testing with Trpm1(-/-) mice confirmed a mutation in Trpm1. Sequencing identified a nucleotide change in exon 23, converting a highly conserved alanine within the pore domain to threonine (p.A1068T). Consistent with prior studies of Trpm1(-/-) mice, no anatomical changes were noted in the Trpm1(tvrm27/tvrm27) retina. The Trpm1(tvrm27/tvrm27) phenotype is distinguished from that of Trpm1(-/-) by the retention of TRPM1 expression on the dendritic tips of depolarizing bipolar cells (DBCs). While ERG b-wave amplitudes of Trpm1(+/-) heterozygotes are comparable to wild type, those of Trpm1(+/tvrm27) mice are reduced by 32%. A similar reduction in the response of Trpm1(+/tvrm27) DBCs to LY341495 or capsaicin is evident in whole cell recordings. These data indicate that the p.A1068T mutant TRPM1 acts as a dominant negative with respect to TRPM1 channel function. Furthermore, these data indicate that the number of functional TRPM1 channels at the DBC dendritic tips is a key factor in defining DBC response amplitude. The Trpm1(tvrm27/tvrm27) mutant will be useful for elucidating the role of TRPM1 in DBC signal transduction, for determining how Trpm1 mutations impact central visual processing, and for evaluating experimental therapies for cCSNB.
Figures




Similar articles
-
A missense mutation in Grm6 reduces but does not eliminate mGluR6 expression or rod depolarizing bipolar cell function.J Neurophysiol. 2017 Aug 1;118(2):845-854. doi: 10.1152/jn.00888.2016. Epub 2017 May 10. J Neurophysiol. 2017. PMID: 28490646 Free PMC article.
-
Congenital stationary night blindness in a patient with mild learning disability due to a compound heterozygous microdeletion of 15q13 and a missense mutation in TRPM1.Ophthalmic Genet. 2021 Jun;42(3):296-299. doi: 10.1080/13816810.2021.1897846. Epub 2021 Mar 10. Ophthalmic Genet. 2021. PMID: 33691579
-
Presentation of TRPM1-Associated Congenital Stationary Night Blindness in Children.JAMA Ophthalmol. 2018 Apr 1;136(4):389-398. doi: 10.1001/jamaophthalmol.2018.0185. JAMA Ophthalmol. 2018. PMID: 29522070 Free PMC article.
-
Properties and functions of TRPM1 channels in the dendritic tips of retinal ON-bipolar cells.Eur J Cell Biol. 2015 Jul-Sep;94(7-9):420-7. doi: 10.1016/j.ejcb.2015.06.005. Epub 2015 Jun 3. Eur J Cell Biol. 2015. PMID: 26111660 Review.
-
Congenital stationary night blindness: an update and review of the disease spectrum in Saudi Arabia.Acta Ophthalmol. 2021 Sep;99(6):581-591. doi: 10.1111/aos.14693. Epub 2020 Dec 26. Acta Ophthalmol. 2021. PMID: 33369259 Review.
Cited by
-
Voriconazole, an antifungal triazol that causes visual side effects, is an inhibitor of TRPM1 and TRPM3 channels.Invest Ophthalmol Vis Sci. 2015 Feb 3;56(2):1367-73. doi: 10.1167/iovs.14-15270. Invest Ophthalmol Vis Sci. 2015. PMID: 25650413 Free PMC article.
-
Identification of a new mutant allele, Grm6(nob7), for complete congenital stationary night blindness.Vis Neurosci. 2015 Jan;32:E004. doi: 10.1017/S0952523815000012. Vis Neurosci. 2015. PMID: 26241901 Free PMC article.
-
A mutagenesis-derived Lrp5 mouse mutant with abnormal retinal vasculature and low bone mineral density.Mol Vis. 2017 Mar 18;23:140-148. eCollection 2017. Mol Vis. 2017. PMID: 28356706 Free PMC article.
-
Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter.EMBO Mol Med. 2014 Sep;6(9):1175-90. doi: 10.15252/emmm.201404077. EMBO Mol Med. 2014. PMID: 25092770 Free PMC article.
-
A Large Endoplasmic Reticulum-Resident Pool of TRPM1 in Retinal ON-Bipolar Cells.eNeuro. 2018 Jul 4;5(3):ENEURO.0143-18.2018. doi: 10.1523/ENEURO.0143-18.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 30027108 Free PMC article.
References
-
- Audo I, Kohl S, Leroy BP, Munier FL, Guillonneau X, Mohand-Saïd S, Bujakowska K, Nandrot EF, Lorenz B, Preising M, Kellner U, Renner AB, Bernd A, Antonio A, Moskova-Doumanova V, Lancelot ME, Poloschek CM, Drumare I, Defoort-Dhellemmes S, Wissinger B, Léveillard T, Hamel CP, Schorderet DF, De Baere E, Berger W, Jacobson SG, Zrenner E, Sahel JA, Bhattacharya SS, Zeitz C. TRPM1 is mutated in patients with autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 85: 720–729, 2009 - PMC - PubMed
-
- Audo I, Bujakowska K, Orhan E, Poloschek CM, Defoort-Dhellemmes S, Drumare I, Kohl S, Luu TD, Lecompte O, Zrenner E, Lancelot ME, Antonio A, Germain A, Michiels C, Audier C, Letexier M, Saraiva JP, Leroy BP, Munier FL, Mohand-Saïd S, Lorenz B, Friedburg C, Preising M, Kellner U, Renner AB, Moskova-Doumanova V, Berger W, Wissinger B, Hamel CP, Schorderet DF, De Baere E, Sharon D, Banin E, Jacobson SG, Bonneau D, Zanlonghi X, Le Meur G, Casteels I, Koenekoop R, Long VW, Meire F, Prescott K, de Ravel T, Simmons I, Nguyen H, Dollfus H, Poch O, Léveillard T, Nguyen-Ba-Charvet K, Sahel JA, Bhattacharya SS, Zeitz C. Whole-exome sequencing identifies mutations in GPR179 leading to autosomal-recessive complete congenital stationary night blindness. Am J Hum Genet 90: 321–330, 2012 - PMC - PubMed
-
- Ball SL, Pardue MT, McCall MA, Gregg RG, Peachey NS. Immunohistochemical analysis of the outer plexiform layer in the nob mouse shows no abnormalities. Vis Neurosci 20: 267–272, 2003 - PubMed
-
- Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci 43: 1595–1603, 2002 - PubMed
-
- Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem 38: 1383–1388, 1990 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

