Neurochemical and neurostructural plasticity in alcoholism
- PMID: 22896799
- PMCID: PMC3419452
- DOI: 10.1021/cn300013p
Neurochemical and neurostructural plasticity in alcoholism
Abstract
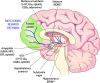
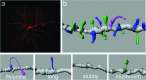
The behavioral manifestations of alcoholism are primarily attributable to the numerous and lasting adaptations that occur in the brain as a result of chronic heavy alcohol consumption. As will be reviewed here, these adaptations include alcohol-induced plasticity in chemical neurotransmission, density and morphology of dendritic spines, as well as neurodegeneration and cerebral atrophy. Within the context of these neuroadaptations that have been observed in both human and animal studies, we will discuss how these changes potentially contribute to the cognitive and behavioral dysfunctions that are hallmark features of alcoholism, as well as how they reveal novel potential pharmacological targets for the treatment of this disorder.
Figures

References
-
- Spanagel R. (2009) Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol. Rev. 89, 649–705. - PubMed
-
- Kiefer F.; Mann K. (2010) Acamprosate: how, where, and for whom does it work? Mechanism of action, treatment targets, and individualized therapy. Curr. Pharm. Des. 16, 2098–2102. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

