Randomized, Crossover and Single-Dose Bioquivalence Study of Two Oral Desogestrel Formulations (Film-Coated Tablets of 75 μg) in Healthy Female Volunteers
- PMID: 22896827
- PMCID: PMC3383212
- DOI: 10.3797/scipharm.1111-18
Randomized, Crossover and Single-Dose Bioquivalence Study of Two Oral Desogestrel Formulations (Film-Coated Tablets of 75 μg) in Healthy Female Volunteers
Abstract
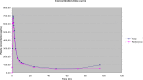
Despite the increase in the substitution of branded medicinal product with generic drugs, this is a controversial issue for some pharmacological groups (such as contraceptives).The aim of the present clinical trial was to assess the bioequivalence and tolerability of two oral formulations of desogestrel.Thirty-three healthy female volunteers participated in this randomized and two-way crossover study. During two separate experimental periods, with at least four weeks of washout period, women received a single oral dose of 75 μg of desogestrel from each of the formulations (test formulation and reference formulation). Desogestrel bioavailability was determined by the measurement of 3-ketodesogestrel plasma concentration.Pharmacokinetic parameters were comparable and the 90% CI for the ratio of C(max) (96.14-114.53%) and AUC(0-t) (105.73-123.83%) values for the test and reference formulations fell within the established regulatory interval (80-125%). Both formulations were also comparable in terms of tolerability.From the results of this study it can be concluded that test formulation (desogestrel 75 μg, Cyndea PHARMA S.L.) is bioequivalent to the reference formulation (Cerazet® 75 μg, Organon Española S.A.).
Keywords: 3-Ketodesogestrel; Adverse events; Bioequivalence; Desogestrel; Low-dose oral contraceptives; Progestogen-only pills.
Figures
Similar articles
-
Comparative bioavailability and pharmacokinetics of investigational enteric- and film-coated formulations of flurbiprofen 100-mg tablets: a single-dose, randomized, open-label, two-period, two-way crossover study in healthy Pakistani male volunteers.Clin Ther. 2010 Mar;32(3):607-13. doi: 10.1016/j.clinthera.2010.03.009. Clin Ther. 2010. PMID: 20399997 Clinical Trial.
-
Bioequivalence of single 100-mg doses of two oral formulations of topiramate: an open-label, randomized-sequence, two-period crossover study in healthy adult male Mexican volunteers.Clin Ther. 2009 Feb;31(2):411-7. doi: 10.1016/j.clinthera.2009.02.001. Clin Ther. 2009. PMID: 19302913 Clinical Trial.
-
Pharmacokinetics and bioequivalence of two formulations of rebamipide 100-mg tablets: a randomized, single-dose, two-period, two-sequence crossover study in healthy Korean male volunteers.Clin Ther. 2009 Nov;31(11):2712-21. doi: 10.1016/j.clinthera.2009.11.010. Clin Ther. 2009. PMID: 20110013 Clinical Trial.
-
Pharmacokinetics and bioequivalence study of three oral formulations of valsartan 160 mg: a single-dose, randomized, open-label, three-period crossover comparison in healthy Indian male volunteers.Clin Ther. 2010 Mar;32(3):588-96. doi: 10.1016/j.clinthera.2010.03.004. Clin Ther. 2010. PMID: 20399995 Clinical Trial.
-
Relative bioavailability and pharmacokinetic properties of two different enteric formulations of esomeprazole in healthy Bangladeshi male volunteers: An open-label, single-dose, randomized-sequence, two-way crossover study.Clin Ther. 2010 Jul;32(7):1419-26. doi: 10.1016/j.clinthera.2010.07.007. Clin Ther. 2010. PMID: 20678688 Clinical Trial.
Cited by
-
Hormonal Contraception and HIV-1 Acquisition: Biological Mechanisms.Endocr Rev. 2018 Feb 1;39(1):36-78. doi: 10.1210/er.2017-00103. Endocr Rev. 2018. PMID: 29309550 Free PMC article. Review.
References
-
- Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update. 2006;12:169–78. http://dx.doi.org/10.1093/humupd/dmi046. - DOI - PubMed
-
- Bergink EW, Hamburger AD, De Jager E, Van der Vies J. Binding of a contraceptive progestogen Org 2969 and its metabolites to receptor proteins and human sex hormone binding globulin. J Steroid Biochem. 1981;14:175–183. http://dx.doi.org/10.1016/0022-4731(81)90171-0. - DOI - PubMed
-
- Kloosterboer HJ, Vonk-Noordegraaf CA, Turpijn EW. Selectivity in progesterone and androgen receptor binding of progestogens used in oral contraception. Contraception. 1988;38:325–332. http://dx.doi.org/10.1016/0010-7824(88)90104-7. - DOI - PubMed
-
- Benagiano G, Primiero FM. Seventhy-five microgram desogestrel minipill, a new perspective in estrogen-free contraception. Ann N Y Acad Sci. 2003;997:163–173. http://dx.doi.org/10.1196/annals.1290.019. - DOI - PubMed
-
- Rice C, Killick S, Hickling D, Coelingh Bennink H. Ovarian activity and vaginal bleeding patterns with a desogestrel-only preparation at three different doses. Hum Reprod. 1996;11:737–740. http://www.ncbi.nlm.nih.gov/pubmed/8671319. - PubMed
LinkOut - more resources
Full Text Sources