Red blood cell endothelial nitric oxide synthase does not modulate red blood cell storage hemolysis
- PMID: 22897637
- PMCID: PMC4313879
- DOI: 10.1111/j.1537-2995.2012.03850.x
Red blood cell endothelial nitric oxide synthase does not modulate red blood cell storage hemolysis
Abstract
Background: The red blood cell (RBC) endothelial nitric oxide synthase (eNOS) has been shown to regulate intrinsic RBC rheologic properties, such as membrane deformability, suggesting that a functional eNOS could be important in RBC viability and function during storage. This study examines the correlation between RBC eNOS deficiency and the propensity of RBCs to hemolyze under selected stress conditions including prolonged hypothermic storage.
Study design and methods: Fresh or stored RBCs from normal and eNOS knockout (KO) mice or from healthy human volunteers were subjected to selected hemolytic stress conditions including mechanical stress hemolysis, osmotic stress hemolysis, and oxidation stress hemolysis and evaluated during standard storage in CPDA-1 solutions.
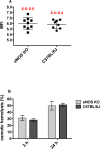
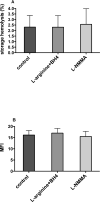
Results: Fresh RBCs from normal and eNOS KO mice demonstrated comparable susceptibility to hemolysis triggered by mechanical stress (mechanical fragility index 6.5 ± 0.5 in eNOS KO vs. 6.4 ± 0.4 for controls; n = 8-9), osmotic stress, and oxidative stress. Additionally, RBCs from both mouse groups exhibited similar hemolytic profile at the end of 14-day hypothermic storage, analogous to 42 days of human RBC storage. Storage of human RBCs (28 days in CPDA-1) in the presence of NOS cofactors (L-arginine and tetrahydro-L-biopterin) or inhibitor (N(5) -[imino(methylamino)methyl]-L-ornithine monoacetate) did not affect cell recovery or hemolytic response to the selected stressors.
Conclusion: These studies suggest that RBC eNOS does not modulate susceptibility to hemolysis in response to selected stress conditions or prolonged hypothermic storage. Other strategies to increase nitric oxide (NO) bioactivity after prolonged storage utilizing NOS-independent pathways such as the nitrate-nitrite-NO pathway may prove a more promising approach.
© 2012 American Association of Blood Banks.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest relevant to this paper
Figures







References
-
- Ozuyaman B, Grau M, Kelm M, Merx MW, Kleinbongard P. RBC NOS: regulatory mechanisms and therapeutic aspects. Trends Mol Med. 2008;14:314–22. - PubMed
-
- Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, Schnurch HG, Godecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rosen P, Kelm M. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–51. - PubMed
-
- Rifkind JM, Nagababu E, Ramasamy S. Nitric oxide redox reactions and red cell biology. Antioxid Redox Signal. 2006;8:1193–203. - PubMed
-
- Bor-Kucukatay M, Wenby RB, Meiselman HJ, Baskurt OK. Effects of nitric oxide on red blood cell deformability. Am J Physiol Heart Circ Physiol. 2003;284:H1577–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

