Redox control of cardiac excitability
- PMID: 22897788
- PMCID: PMC3526898
- DOI: 10.1089/ars.2011.4234
Redox control of cardiac excitability
Abstract
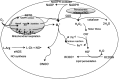
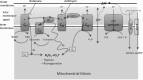
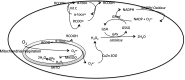
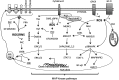
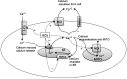
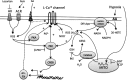
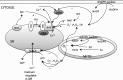
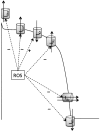
Reactive oxygen species (ROS) have been associated with various human diseases, and considerable attention has been paid to investigate their physiological effects. Various ROS are synthesized in the mitochondria and accumulate in the cytoplasm if the cellular antioxidant defense mechanism fails. The critical balance of this ROS synthesis and antioxidant defense systems is termed the redox system of the cell. Various cardiovascular diseases have also been affected by redox to different degrees. ROS have been indicated as both detrimental and protective, via different cellular pathways, for cardiac myocyte functions, electrophysiology, and pharmacology. Mostly, the ROS functions depend on the type and amount of ROS synthesized. While the literature clearly indicates ROS effects on cardiac contractility, their effects on cardiac excitability are relatively under appreciated. Cardiac excitability depends on the functions of various cardiac sarcolemal or mitochondrial ion channels carrying various depolarizing or repolarizing currents that also maintain cellular ionic homeostasis. ROS alter the functions of these ion channels to various degrees to determine excitability by affecting the cellular resting potential and the morphology of the cardiac action potential. Thus, redox balance regulates cardiac excitability, and under pathological regulation, may alter action potential propagation to cause arrhythmia. Understanding how redox affects cellular excitability may lead to potential prophylaxis or treatment for various arrhythmias. This review will focus on the studies of redox and cardiac excitation.
Figures


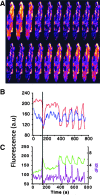
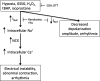
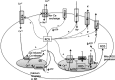
Similar articles
-
Redox regulation of sodium and calcium handling.Antioxid Redox Signal. 2013 Mar 20;18(9):1063-77. doi: 10.1089/ars.2012.4818. Epub 2012 Oct 3. Antioxid Redox Signal. 2013. PMID: 22900788 Free PMC article. Review.
-
Mitochondria and arrhythmias.Free Radic Biol Med. 2014 Jun;71:351-361. doi: 10.1016/j.freeradbiomed.2014.03.033. Epub 2014 Apr 5. Free Radic Biol Med. 2014. PMID: 24713422 Free PMC article. Review.
-
Mitochondria-derived ROS bursts disturb Ca²⁺ cycling and induce abnormal automaticity in guinea pig cardiomyocytes: a theoretical study.Am J Physiol Heart Circ Physiol. 2015 Mar 15;308(6):H623-36. doi: 10.1152/ajpheart.00493.2014. Epub 2014 Dec 24. Am J Physiol Heart Circ Physiol. 2015. PMID: 25539710 Free PMC article.
-
Mitochondrial reactive oxygen species production and elimination.J Mol Cell Cardiol. 2014 Aug;73:26-33. doi: 10.1016/j.yjmcc.2014.03.011. Epub 2014 Mar 20. J Mol Cell Cardiol. 2014. PMID: 24657720 Review.
-
Gender disparities in cardiac cellular electrophysiology and arrhythmia susceptibility in human failing ventricular myocytes.Int Heart J. 2005 Nov;46(6):1105-18. doi: 10.1536/ihj.46.1105. Int Heart J. 2005. PMID: 16394606
Cited by
-
Salinomycin induced ROS results in abortive autophagy and leads to regulated necrosis in glioblastoma.Oncotarget. 2016 May 24;7(21):30626-41. doi: 10.18632/oncotarget.8905. Oncotarget. 2016. PMID: 27121320 Free PMC article.
-
Circadian redox rhythms in the regulation of neuronal excitability.Free Radic Biol Med. 2018 May 1;119:45-55. doi: 10.1016/j.freeradbiomed.2018.01.025. Epub 2018 Feb 2. Free Radic Biol Med. 2018. PMID: 29398284 Free PMC article. Review.
-
SERCA2a upregulation ameliorates cellular alternans induced by metabolic inhibition.J Appl Physiol (1985). 2016 Apr 15;120(8):865-75. doi: 10.1152/japplphysiol.00588.2015. Epub 2016 Feb 4. J Appl Physiol (1985). 2016. PMID: 26846549 Free PMC article.
-
The Actions of Lyophilized Apple Peel on the Electrical Activity and Organization of the Ventricular Syncytium of the Hearts of Diabetic Rats.J Diabetes Res. 2016;2016:8178936. doi: 10.1155/2016/8178936. Epub 2015 Dec 29. J Diabetes Res. 2016. PMID: 26839897 Free PMC article.
-
Cardiac Tissue Engineering: A Journey from Scaffold Fabrication to In Vitro Characterization.Small Sci. 2024 Jul 22;4(9):2400079. doi: 10.1002/smsc.202400079. eCollection 2024 Sep. Small Sci. 2024. PMID: 40212070 Free PMC article.
References
-
- Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. - PubMed
-
- Adachi T. Weisbrod RM. Pimentel DR. Ying J. Sharov VS. Schoneich C. Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. - PubMed
-
- Ahern GP. Hsu SF. Klyachko VA. Jackson MB. Induction of persistent sodium current by exogenous and endogenous nitric oxide. J Biol Chem. 2000;275:28810–28815. - PubMed
-
- Ahmmed GU. Xu Y. Hong Dong P. Zhang Z. Eiserich J. Chiamvimonvat N. Nitric oxide modulates cardiac Na(+) channel via protein kinase A and protein kinase G. Circ Res. 2001;89:1005–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

