Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels
- PMID: 22897853
- PMCID: PMC3422514
- DOI: 10.1016/j.ccr.2012.06.013
Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels
Abstract
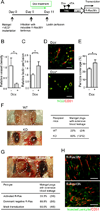
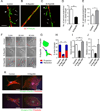
We show that R-Ras, a small GTPase of the Ras family, is essential for the establishment of mature, functional blood vessels in tumors. The genetic disruption of R-Ras severely impaired the maturation processes of tumor vessels in mice. Conversely, the gain of function of R-Ras improved vessel structure and blood perfusion and blocked plasma leakage by enhanced endothelial barrier function and pericyte association with nascent blood vessels. Thus, R-Ras promotes normalization of the tumor vasculature. These findings identify R-Ras as a critical regulator of vessel integrity and function during tumor vascularization.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
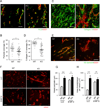
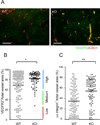
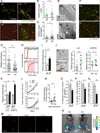
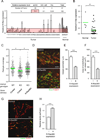


Comment in
-
Angiogenesis: The new normal.Nat Rev Cancer. 2012 Oct;12(10):660-1. doi: 10.1038/nrc3360. Epub 2012 Sep 6. Nat Rev Cancer. 2012. PMID: 22952010 No abstract available.
References
-
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
-
- Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

